COVID-19 Drugs: University Of Virginia Mice Study Demonstrates That Adenosine A2AR Agonist - Apadenoson Significantly Improves COVID-19 Survival Rate!
Thailand Medical News Team Aug 19, 2023 1 year, 8 months, 1 day, 15 hours, 29 minutes ago
COVID-19 Drugs: The ongoing battle against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the COVID-19 pandemic, has driven relentless research to identify effective treatments and interventions.
A pivotal
COVID-19 Drugs study conducted by the University of Virginia School of Medicine has illuminated a potential breakthrough in the form of the adenosine A2AR agonist, Apadenoson. This innovative approach demonstrates remarkable promise in improving clinical symptoms, mitigating cytokine storms, and enhancing survival rates in SARS-CoV-2-infected mice.
Unraveling COVID-19 Challenges
COVID-19 is characterized by its destructive cytokine storm, a hyperinflammatory response leading to hospitalization and the need for supplemental oxygen. Numerous strategies have aimed to mitigate this cytokine storm to ameliorate patient outcomes. One of the emerging avenues in this endeavor involves adenosine A2AR agonists, which exhibit the potential to dampen cytokine release from various immune cells.
The Power of Apadenoson
At the forefront of these efforts, Apadenoson, a potent and selective adenosine analog with robust anti-inflammatory properties, has emerged as a game-changer. Administered via subcutaneous osmotic pumps to SARS-CoV-2-infected mice, Apadenoson showcases an impressive range of positive effects. These include a reduction in weight loss, improved clinical symptoms, decreased levels of proinflammatory cytokines and chemokines in bronchial lavage fluid, and significantly enhanced survival rates among the K18-hACE2 transgenic mice.
Adenosine's Immune Modulation
Adenosine, a key regulator of the immune response, interacts with four G protein-coupled receptors - A1, A2A, A2B, and A3. A2AR agonists offer a particularly promising avenue due to their ability to inhibit cytokine production and platelet activation. Activation of A2AR receptors triggers a cascade of events that result in the suppression of NF-kB-mediated transcription of inflammatory cytokines. Notably, A2AR agonists have demonstrated effectiveness in various respiratory conditions, including asthma and chronic obstructive pulmonary disease (COPD), by limiting leukocyte influx and reducing inflammation.
Translating Success: Apadenoson's Journey
The success of Apadenoson isn't limited to COVID-19. Previous studies have indicated its potential to enhance survival rates in models of sepsis, reduce joint inflammation in septic arthritis, and protect lungs from ischemia-reperfusion and transplant injuries. This diverse spectrum of benefits stems from Apadenoson's ability to suppress antibiotic-induced cytokine storms while maintaining bacterial clearance. These findings provided the impetus to explore its potential against SARS-CoV-2 infection.
Clinical Implications and Future Prospects
The University of Virginia's study on Apadenoson showcases the potential to revolutionize COVID-19 treatment paradigms. By curbing the inflammatory response and enhancing survival rates, this
novel approach holds promise not only for improving acute COVID-19 cases but also for reducing the incidence of long COVID-19, which is associated with prolonged inflammation.
Fine-Tuning Treatment Strategies
The research indicated that Apadenoson's administration after infection yielded more favorable outcomes compared to pre-infection treatment. This suggests a nuanced approach where Apadenoson could play a pivotal role in tempering early immune responses and curbing the later cytokine storm that becomes life-threatening. This dual action underscores the complexity of immune modulation and the importance of timing in therapeutic strategies.
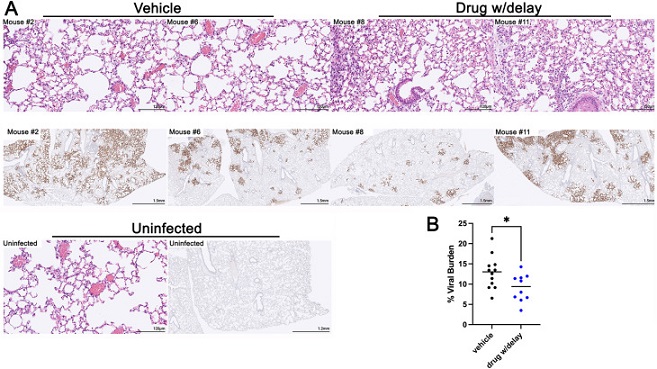 Less Viral burden on day five post-infection in mice treated therapeutically with Apadenoson. A) Lungs were stained with H&E or a SARS CoV-2 anti-NP antibody. Representative mice are shown. Mice #2 and 6 were vehicle-treated; Mice #8 and #11 were therapeutically (drug with delay, 5 h after infection) -treated. H&E-stained lungs are shown at a higher magnification than IHC slides. B) Percentage of viral staining in the lungs by treatment group. P value = 0.0298, unpaired t-test. The percent viral burden in the lungs was determined using ImageJ. These results are combined from two independent experiments
Viral Burden Reduction
Less Viral burden on day five post-infection in mice treated therapeutically with Apadenoson. A) Lungs were stained with H&E or a SARS CoV-2 anti-NP antibody. Representative mice are shown. Mice #2 and 6 were vehicle-treated; Mice #8 and #11 were therapeutically (drug with delay, 5 h after infection) -treated. H&E-stained lungs are shown at a higher magnification than IHC slides. B) Percentage of viral staining in the lungs by treatment group. P value = 0.0298, unpaired t-test. The percent viral burden in the lungs was determined using ImageJ. These results are combined from two independent experiments
Viral Burden Reduction
Apadenoson's influence extended beyond cytokine modulation, as it was also found to reduce viral burden in the lungs. The mechanism behind this phenomenon remains a subject of investigation, but it is speculated that reduced inflammation may mitigate immune exhaustion and apoptosis, ultimately curbing the viral load.
Variants and Future Research
While the study primarily focused on one strain of SARS-CoV-2, it raises questions about Apadenoson's effectiveness against different variants. Variability in virulence and immune response among variants could potentially impact the drug's efficacy, necessitating further exploration and adaptation of treatment strategies.
Conclusion
In a landscape fraught with challenges, the University of Virginia's mice study on Apadenoson shines as a beacon of hope. Its ability to alleviate cytokine storms, enhance survival rates, and reduce viral burden demonstrates its potential as a transformative COVID-19 treatment. While further research is essential, Apadenoson's journey from the lab to potential clinical use represents a remarkable stride towards conquering this global pandemic.
The study findings were published in the peer reviewed journal: Heliyon.
https://www.cell.com/heliyon/fulltext/S2405-8440(23)06434-4
For the latest on
COVID-19 Drugs, keep on logging to Thailand Medical News.

