COVID-19 Immunology: Study Shows That Early Exposure of Nasopharyngeal Mucosa To Interferon Activates Epithelial Cells To Inhibit SARS-CoV-2
Source: COVID-19 Immunology Sep 02, 2021 4 years, 3 months, 1 week, 3 days, 1 hour, 44 minutes ago
COVID-19 Immunology: A new study by the University of Vienna-Austria has shown that early exposure of the human host’s nasopharyngeal mucosa to antiviral interferon activates epithelial cells to inhibit the SARS-CoV-2 coronavirus.
The study findings were published in the peer reviewed the Journal of Experimental Medicine (JEM).
https://rupress.org/jem/article/218/10/e20211667/212589/The-early-interferon-catches-the-SARS-CoV-2IFN
The study focuses on the importance of interferon (IFN) in severe acute respiratory syndromes coronavirus-2 (SARS-CoV-2) infection.
The research was based on earlier critical findings from two other published studies that revealed that a suitable amount of antiviral IFN activates epithelial cells of the nasopharyngeal mucosa to inhibit SARS-CoV-2 growth. These IFN-induced mucosal genes could serve as biomarkers of the COVID-19 disease.
https://rupress.org/jem/article/218/10/e20211211/212540/Early-nasal-type-I-IFN-immunity-against-SARS-CoV-2
https://rupress.org/jem/article/218/8/e20210583/212380/Dynamic-innate-immune-response-determines
Typically the key role of interferon or IFN is to protect against viral invasions. In general the type I IFN, which includes IFNα, β, and ω, as well as type III IFN, are associated with antiviral immunity.
It is known that IFN-I receptors are present in all somatic cells. Comparatively, IFN-III receptors show tissue-restricted expression and are mostly found in the epithelial cells, which support innate immunity at the viral entry site.
Also both IFN-I and IFN-III receptors culminate in the activation of a master transcription factor ISGF3. ISGF3 is composed of the subunits STAT1, STAT2, and IRF9. ISGF3 regulates the induction of IFN-induced genes (ISG), which form a cell-autonomous antiviral state.
The efficacy of IFN in treating COVID-19 has been moderate. Medical researchers believe that in order to increase the success rate of IFN treatment, a detailed understanding of the role of endogenous IFN production during infection is essential.
Past studies have identified several factors that hinder the effectiveness of IFN. Similar to many coronaviruses, the SARS-CoV-2 genome also expresses factors that inhibit IFN synthesis and response.
The study team has also revealed that some hosts with genetic disorders inhibit the production of IFN.
Alarmingly in some cases, the synthesis of IFN-neutralizing autoantibodies occurs as a result of a genetic anomaly that reduces IFN efficiency.
Previous
COVID-19 Immunology studies have also revealed that the proinflammatory character of IFN-I often aggravates the course of advanced disease, especially through the activati
on of immune cells like monocytes.
It’s critical to understand the limiting factors imposed by both the host as well as the virus that threaten IFN-related protective mechanisms at the nasopharyngeal mucosae. This is important because SARS-CoV-2 infects the upper respiratory tract of the host and viral replication at this site is associated with its transmission.
The initial two studies published in the JEM of which this study was based on revealed early events of SARS-CoV-2 infection by analyzing both nasal swabs of COVID-19 patients, as well as cellular models of nasopharyngeal epithelium. Upon comparing the results of COVID-19 infected and non-infected healthcare workers, researchers observed the presence of ISG signatures and their dynamics in blood leukocytes in nasal samples collected during the early stage of infection.
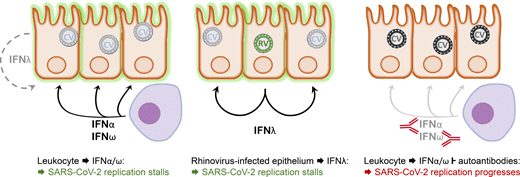 Model depicting the interaction between SARS-CoV-2 and the nasopharyngeal epithelium. Left: In a situation of asymptomatic infection or mild disease, mucosal leukocytes provide the IFN-I IFNα and IFNω for an inhibitory antiviral state. IFNλ production by the infected cells alone is insufficient in this situation. Middle: Infection with an RNA virus such as rhinovirus causes epithelial cells to produce sufficient IFNλ to cause an antiviral state in bystander cells that subsequently lose permissiveness for SARS-CoV-2 replication. Right: As in the left panel, but autoantibodies inhibit the leukocyte-derived IFN. Consequently, the epithelium remains permissive for SARS-CoV-2 replication, allowing for virus spread to the lower respiratory tract and favoring the development of severe pulmonary disease.
Model depicting the interaction between SARS-CoV-2 and the nasopharyngeal epithelium. Left: In a situation of asymptomatic infection or mild disease, mucosal leukocytes provide the IFN-I IFNα and IFNω for an inhibitory antiviral state. IFNλ production by the infected cells alone is insufficient in this situation. Middle: Infection with an RNA virus such as rhinovirus causes epithelial cells to produce sufficient IFNλ to cause an antiviral state in bystander cells that subsequently lose permissiveness for SARS-CoV-2 replication. Right: As in the left panel, but autoantibodies inhibit the leukocyte-derived IFN. Consequently, the epithelium remains permissive for SARS-CoV-2 replication, allowing for virus spread to the lower respiratory tract and favoring the development of severe pulmonary disease.
Interestingly both studies revealed that the accurate measurement of the expression of selected ISGs could be a potential way to detect early nasopharyngeal viral replication.
The French study established an ISG score set by the expression of four ISGs; namely, IFI27, IFI44L, RSAD2, and IFIT1.
https://rupress.org/jem/article/218/10/e20211211/212540/Early-nasal-type-I-IFN-immunity-against-SARS-CoV-2
But the American study by Yale University indicated that measuring the level of chemokine CXCL10 is an adequate method to determine viral loads.
https://rupress.org/jem/article/218/8/e20210583/212380/Dynamic-innate-immune-response-determines
The current study team is highly optimistic about these results and believes that this approach opens a new avenue for the detection of early SARS-CoV-2 infection. The viral load could be determined by analyzing the replication rate of different viral variants.
Importantly both studies provided detailed evidence of mucosal innate immunity to SARS-CoV-2, which could help physicians plan proper treatment strategies.
Also, these studies determined disease parameters by studying severely infected COVID-19 patients with autoantibodies to IFN-I.
It was found that when the sera completely neutralized IFN-I (IFNα2 and IFNω), the nasopharyngeal epithelia expressed low ISG scores, despite high viral loads. This might be due to the impaired innate immune response to SARS-CoV-2, which may be an appropriate indicator for the administration of antiviral drugs.
The current study team also revealed that treatment of infected cells with IFNα2 blocks viral replication. Antiviral effects and ISG induction are also blocked in serum-containing anti-IFN autoantibodies.
The Austrian study shows that the IFN response of the mucosae of the upper respiratory tract can inhibit SARS-CoV-2 replication. Scientists agree that the epithelial IFNλ production is not enough to restrict SARS-CoV-2 replication.
Herein, the study team has identified IFNα2 and IFNω as prime mediators of innate antiviral effects. Therefore, the team suggests that leukocytes are required for the production of an antiviral state in epithelial cells. The study team further proposes that IFN treatment at an early stage of SARS-CoV-2 infection could inhibit viral spread to the lower respiratory tract which would, in turn, prevent the onset of pulmonary disease.
The study findings indicate that both IFNα2 and IFNω possess potential antiviral properties. Although several studies are available that elucidate the role of IFNα, limited studies have been focused on the production of IFNω in humans. More research is therefore required to understand the role of IFNω during SARS-CoV-2 infection and the impact of this cytokine in innate immunity against other respiratory viruses.
For the latest on
COVID-19 Immunology, keep on logging to Thailand Medical News.

