COVID-19 Infections Causes Reprogramming Of Leukocyte Metabolism By The Activation Of Certain Amino Acid Pathways
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 25, 2024 1 year, 2 months, 1 day, 2 hours, 7 minutes ago
COVID-19 News: The COVID-19 pandemic has ushered in an era of intensive research aimed at deciphering the multifaceted interactions between the SARS-CoV-2 virus and the human immune system. At the forefront of this exploration is a groundbreaking study covered in this
COVID-19 News report, conducted by researchers at the University of Tuscia in Italy, shedding light on the intricate metabolic changes within leukocytes. This investigation has unveiled a novel perspective on how amino acid pathways are reprogrammed post-SARS-CoV2 infection, influencing immune memory and potentially paving the way for innovative therapeutic interventions.
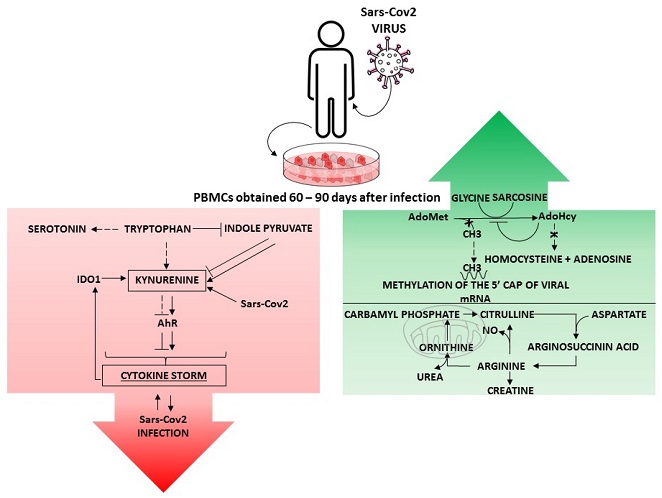 Amino Acid Metabolism in Leukocytes Showing In Vitro IgG Memory from SARS-CoV2-Infected Patients
Differentially regulated signaling pathways in PBMCs exhibiting IgG antibody memory against SARS-CoV2. The green box shows the methionine and arginine metabolism pathways that were upregulated after infection, whereas the red box shows the tryptophan pathway that was downregulated in IgGm+ samples. Taken together, these pathways were involved in physiological recovery from viral infection and immune activity [20,22]. S-adenosylmethionine (AdoMet); S-adenosyl-L-homocysteine (AdoHcy); indoleamine 2,3-dioxygenase 1 (IDO1); aryl hydrocarbon receptor (AhR).
Metabolic Complexity in SARS-CoV2-Infected Patients
Amino Acid Metabolism in Leukocytes Showing In Vitro IgG Memory from SARS-CoV2-Infected Patients
Differentially regulated signaling pathways in PBMCs exhibiting IgG antibody memory against SARS-CoV2. The green box shows the methionine and arginine metabolism pathways that were upregulated after infection, whereas the red box shows the tryptophan pathway that was downregulated in IgGm+ samples. Taken together, these pathways were involved in physiological recovery from viral infection and immune activity [20,22]. S-adenosylmethionine (AdoMet); S-adenosyl-L-homocysteine (AdoHcy); indoleamine 2,3-dioxygenase 1 (IDO1); aryl hydrocarbon receptor (AhR).
Metabolic Complexity in SARS-CoV2-Infected Patients
SARS-CoV2, belonging to the beta-coronavirus strain B1-3, has manifested as a clinical enigma, presenting a spectrum of symptoms ranging from mild to severe. Variants such as Alpha, Delta, and Omicron have introduced new challenges, each with its distinct transmissibility and severity profiles. The severity of COVID-19 is exacerbated in individuals with pre-existing risk factors, emphasizing the need to understand the intricate dynamics of the immune response.
The immune response to SARS-CoV2 is a dynamic interplay of innate and specific components, involving B and T cells. Unraveling this response is crucial for effective virus management and prevention. Furthermore, gaining insights into the molecular mechanisms governing physiological recovery from viral infections, particularly within leukocytes, is imperative for understanding the antibody-mediated immune response to SARS-CoV2.
The S1 protein on the virus's surface has emerged as a key player, triggering immune responses. Recent research has sought to establish connections between the post-infection stages of SARS-CoV2 and various metabolic pathways within leukocytes.
Amino Acid Metabolism: Orchestrating Immune Responses
Metabolic profiling has become an invaluable tool in unraveling the molecular intricacies of COVID-19 pathophysiology. Within this landscape, amino acid metabolism has assumed a central role, dictating immune responses and influencing viral replication dynamics.
An intriguing study examining the correlation between COVID-19 symptom
severity and amino acid concentrations revealed a nuanced relationship. Severe cases exhibited elevated levels of specific amino acids, hinting at a potential prognostic value. Metabolomics analysis of COVID-19 patients uncovered modifications in amino acid metabolism associated with altered oxygen homeostasis.
Leukocyte Response and IgG Memory: A Unique Perspective
The University of Tuscia's research provides a distinctive lens through which to examine the later phases of immune defense against SARS-CoV2 infection. By focusing on peripheral blood mononuclear cells (PBMCs) with in vitro IgG memory for the spike S1 antigen 60–90 days post-infection, the study introduces a novel approach. Leveraging in vitro PBMC cultures and metabolomics, this research delves into the enduring impact of the virus on leukocyte metabolism.
Methionine Cycle: Unraveling Viral Replication Mechanisms
The methionine cycle emerges as a critical player in leukocyte metabolism post-SARS-CoV2 infection. Methionine, a pivotal amino acid, exhibits the potential to modulate the assembly of SARS-CoV2 by interfering with the virus's RNA polymerase. Intriguingly, the virus appears to exploit the host's folate and one-carbon metabolism for its intracellular replication, unveiling a potential therapeutic target.
Arginine Metabolism: A Delicate Balance Between Immunity and Endothelial Function
Arginine, classified as a non-essential amino acid, assumes a pivotal role in immune responses. The presence of arginase-1 (Arg1) significantly influences antiviral immune responses. COVID-19 patients exhibited altered arginine metabolism, with a higher ratio of L-arginine to ornithine suggesting increased arginase activity. The potential of arginine supplementation to control chronic inflammation and improve endothelial function in COVID-19 patients sparks intriguing possibilities.
Tryptophan Metabolism: A Keystone in Severe COVID-19
Clinical studies have shed light on the selective enhancement of the kynurenine pathway in severe COVID-19 patients. Tryptophan, an essential amino acid, and its metabolites have been shown to modulate the immune system and reduce inflammation. The restoration of kynurenine metabolism in post-infection stages, as seen in PBMCs with IgG memory, introduces a potential avenue for therapeutic intervention.
Conclusion
In summary, the study findings have significantly advanced our understanding of the intricate metabolic changes within leukocytes in response to SARS-CoV2 infection. The reprogramming of amino acid pathways, including the methionine cycle, arginine cycle, and tryptophan cycle, has been unveiled, offering a unique perspective on the enduring impact of the virus on immune memory.
These insights not only contribute to deciphering the immune response but also present promising targets for therapeutic interventions. The intricate interplay between viral pathogenesis and leukocyte metabolism opens up new avenues for further research, fostering advancements in our ability to combat the ongoing COVID-19 pandemic. As we navigate this scientific landscape, the analysis of these metabolites may emerge as a crucial biomarker, offering a glimpse into the effectiveness and longevity of the antiviral activation of PBMCs.
The study findings were published in the peer reviewed journal: Diseases.
https://www.mdpi.com/2079-9721/12/3/43
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
