COVID-19 Medical Devices: US FDA Grants Emergency Use Authorization To VitalPatch For Cardiac Monitoring In COVID-19 Patients
Source: COVID-19 Medical Devices May 18, 2020 4 years, 11 months, 1 week, 1 day, 10 hours, 33 minutes ago
Medical Devices: VitalConnect a Silicon Valley based biotech company has announced that it has received US FDA Emergency Use Authorization status for use of its VitalPatch to detect changes in the QT interval of hospitalized patients undergoing drug treatment for COVID-19.
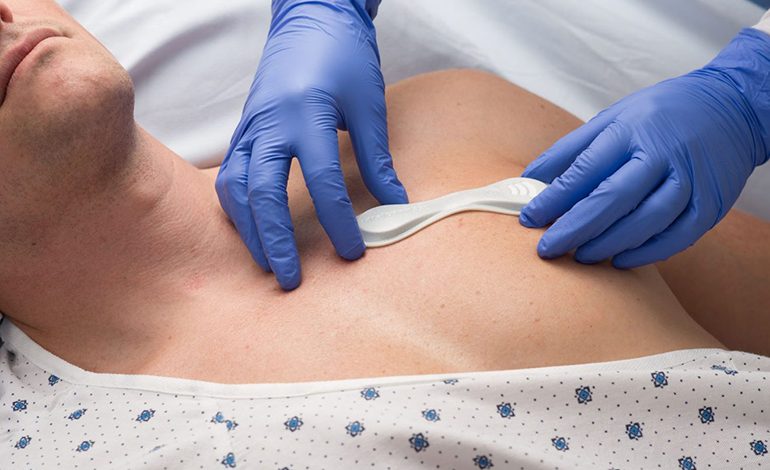
Due to cardio-toxicity of many experimental drugs used to treat COVID-19 patients, many doctors would benefit from this new medical device.
It has been found that Hydroxychloroquine, chloroquine, azithromycin and numerous antivirals used to treat some COVID-19 patients, are associated with risk of prolonged QT interval that can lead to life-threatening arrhythmias.
This new medical device: VitalPatch, allows doctors to remotely and continuously monitor patients at risk of QT prolongation due to COVID-19 treatment.
The new VitalPatch is an US FDA-approved device. Besides serving as a single-lead ECG, it monitors seven other physiological parameters continuously, including body temperature, heart rate, heart rate variability, respiratory rate, and blood oxygen saturation levels. It can also integrate with third-party devices to monitor blood pressure, weight, and oxygen saturation. According to the company, it is the first and only biosensor capable of monitoring such a broad set of patient vitals.

The medical device can be worn for up to seven days and is powered by a disposable zinc air battery with up to 168 hours of battery life. It measures 120 x 40 mm and is secured to the patient by a hydrocolloid adhesive.
Dr Joe Roberson, Chief Medical Officer of VitalConnect told Thailand Medical News, “COVID-19 presents a myriad of symptoms and clinicians need access to medical devices that allow them to monitor and manage those symptoms in real-time in order to create the most appropriate treatment plans for each individual. The enhancement of the VitalPatch receiving Emergency Use Authorization for QT-interval detection will enable this platform to further support clinicians who are on the frontlines of treating this virus.”
For product details, please refer to:
https://vitalconnect.com/about/
For more new and unique
medical devices suitable for use during the COVID-19 crisis, keep logging to Thailand Medical News.
AN URGENT APPEAL: Please help support this website by kindly making a donation to sustain this website and also all in all our initiatives to propel further research: https://www.thailandmedical.news/p/sponsorship

