COVID-19 News: Australian Study Shows That SARS-Cov-2 Omicron BA.5 And XBB Variants Have Increased Neurotropic Potential Over BA.1
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 26, 2023 1 year, 4 months, 3 weeks, 3 days, 13 hours, 58 minutes ago
COVID-19 News: The ever-evolving landscape of the SARS-CoV-2 virus has introduced new challenges in understanding its pathogenicity and potential impact on human health. In a groundbreaking study covered in this
COVID-19 News report, conducted by the QIMR Berghofer Medical Research Institute, University of Queensland, and the Australian Infectious Disease Research Centre in Brisbane-Australia, researchers have shed light on the neurotropic potential of the emerging omicron variants, specifically BA.5 and XBB, in comparison to the previously studied BA.1.
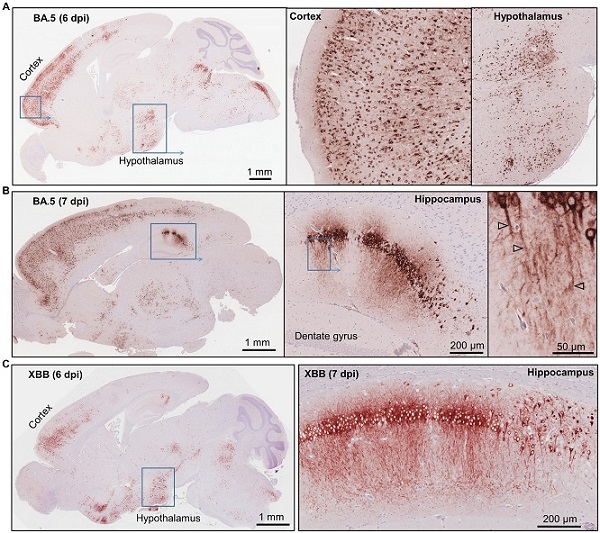 Immunohistochemistry of brains of BA.5 and XBB infected K18-hACE2 mice using an anti-spike monoclonal antibody. (A) Brain of BA.5-infected K18-hACE2 mouse 6 dpi showing IHC staining in the cortex and hypothalamus. Insert enlargements on the right. (B) As for “a” showing staining of the hippocampus 7 dpi. Far right shows staining of the axons (arrowheads). (C) XBB-infected K18-hACE2 mouse showing IHC staining in the cortex and hypothalamus, 6 dpi. Staining of the hippocampus for a mouse euthanized 7 dpi.
Omicron Lineage Dynamics
Immunohistochemistry of brains of BA.5 and XBB infected K18-hACE2 mice using an anti-spike monoclonal antibody. (A) Brain of BA.5-infected K18-hACE2 mouse 6 dpi showing IHC staining in the cortex and hypothalamus. Insert enlargements on the right. (B) As for “a” showing staining of the hippocampus 7 dpi. Far right shows staining of the axons (arrowheads). (C) XBB-infected K18-hACE2 mouse showing IHC staining in the cortex and hypothalamus, 6 dpi. Staining of the hippocampus for a mouse euthanized 7 dpi.
Omicron Lineage Dynamics
The omicron lineage, which emerged as a divergent strain from earlier variants of concern, has raised questions about its evolutionary origins. The BA.5 sub-lineage swiftly became the predominant SARS-CoV-2 virus in various countries, while the XBB variant gained momentum in its transmission. The rapid global spread of omicron variants has outpaced previous strains, marking a distinctive chapter in the ongoing COVID-19 pandemic.
Long-COVID and Neurological Manifestations
As researchers delve deeper into the characteristics of omicron variants, the study underscores the relevance of long-COVID, a phenomenon associated with persistent symptoms post-infection. Neurological and psychiatric manifestations have become increasingly recognized components of both acute COVID-19 and long-COVID. Despite this, specific data on neurological manifestations related to BA.5 and XBB variants are still emerging.
Challenges in Assessing Pathogenicity
The study grapples with the challenge of assessing the pathogenicity of BA.5 and XBB variants compared to their predecessor, BA.1. Conflicting findings from various studies using animal models and human populations have fueled the controversy. Some studies suggest increased lung infection/pathology for BA.5, while others argue that the reduced pathogenicity observed in early omicron variants is retained in BA.5 and XBB.
The K18-hACE2 Mouse Model
To address these controversies and gain insights into the neurotropic potential of omicron variants, the researchers employed the K18-hACE2 mouse model. This model mimics severe COVID-19, replicating lung pathology and inflammatory pathways seen in humans. Intranasal inoculation of original ancestral isolates in these mice results in fulminant and lethal brain infections, highlighting its utility for studying SARS-CoV-2 biology.
Neurotropic Potential of BA.5 and
XBB
The results of the study reveal a significant increase in neurotropic potential for BA.5 and XBB variants compared to BA.1. Notably, BA.5 and XBB isolates demonstrated higher pathogenicity in K18-hACE2 mice, leading to fulminant brain infection and increased mortality. This finding challenges the assumption that the attenuation observed in the BA.1 sub-lineage persists in later omicron variants.
Cellular Targets in Brain Organoids
In addition to the mouse model, the researchers explored the neurotropic potential of BA.5 and XBB in human cortical brain organoids. These 3D mini-brain structures derived from induced pluripotent cells allowed the researchers to observe infection patterns at the cellular level. BA.5 exhibited a greater capacity to infect human cortical brain organoids compared to BA.1, suggesting an escalating neurotropic potential in evolving omicron variants.
Understanding Brain Infection in COVID-19
Neurological abnormalities in COVID-19 patients have been increasingly documented, with encephalitis identified in severe cases. The mechanisms underlying brain pathology and neuropathology remain complex, involving both systemic cytokine storms and potential direct brain infections. Previous studies have reported the presence of viral RNA or protein in the brains of deceased COVID-19 patients.
Histological Insights
Histological examinations of BA.5 and XBB-infected K18-hACE2 mouse brains revealed notable lesions, including neuron vacuolation, perivascular cuffing, and microglial nodules. These observations align with histological findings in post-mortem COVID-19 patient brains. Despite the fulminant brain infection not being a common feature of human COVID-19, shared histological lesions suggest potential similarities in neuropathology.
RNA-Seq Analysis
The researchers conducted RNA-Seq analysis on BA.5-infected K18-hACE2 mouse brains, revealing a cytokine storm characterized by the upregulation of interferons, TNF, IL-1, and IL-6. Bioinformatic analyses also highlighted neuropathology-associated annotations, reinforcing the presence of inflammatory responses reminiscent of acute SARS-CoV-2 infections.
Comparative Analysis with Human COVID-19
To enhance the translational relevance of their findings, the researchers compared cytokine gene expression patterns in BA.5-infected K18-hACE2 mouse brains with data from severe COVID-19 patients. Remarkably, there was a significant correlation, indicating shared inflammatory pathways and cytokine responses between the two species.
Infection of Human Cortical Brain Organoids
The study extended its investigation to human cortical brain organoids, providing insights into the neurovirulence of BA.5. These organoids, derived from induced pluripotent cells, demonstrated BA.5's enhanced capacity to infect a greater number of cells compared to BA.1. RNA-Seq analysis further highlighted the distinctive response of organoids to BA.5 infection, emphasizing the virus's neurotropic properties.
Conclusion
In unraveling the neurotropic potential of SARS-CoV-2 omicron variants, the Australian study offers valuable insights into the evolving dynamics of the virus. The increased neurotropic potential observed in BA.5 and XBB variants in K18-hACE2 mice and human brain organoids underscores the importance of continued vigilance and research. As the COVID-19 pandemic continues, understanding the intricate interactions between the virus and the nervous system becomes crucial for developing targeted interventions and mitigating potential neurological complications associated with emerging variants.
The study findings were published in the peer reviewed journal: Frontiers in Microbiology.
https://www.frontiersin.org/articles/10.3389/fmicb.2023.1320856/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
