COVID-19 News: German And Swiss Study Finds That Human PBMCs Form Lipid Droplets In Response To SARS-CoV-2 Spike Proteins!
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 05, 2023 2 years, 2 months, 1 week, 3 days, 22 hours, 9 minutes ago
COVID-19 News: The ongoing COVID-19 pandemic has continually posed a profound impact on global health and scientific research. In the quest to better understand the virus and its effects on human cells, a recent collaborative study conducted by researchers from Hannover Medical School in Germany, ExcellGene SA in Switzerland, and École Polytechnique Fédérale de Lausanne in Switzerland has shed new light on how SARS-CoV-2 spike proteins influence human peripheral blood mononuclear cells (PBMCs).
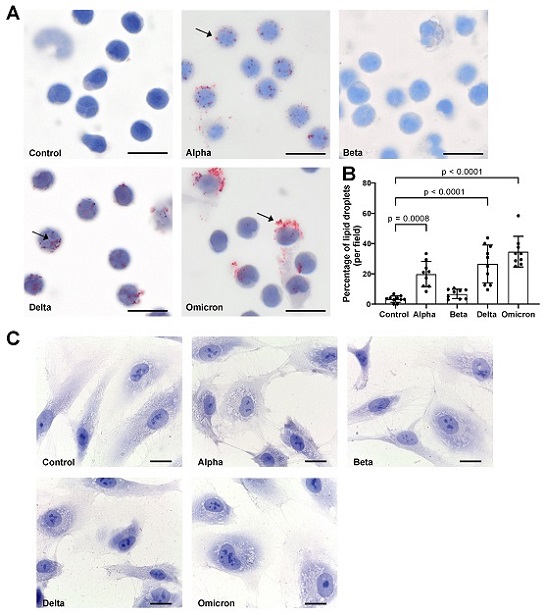 Effects of spike proteins on lipid droplet (LD) formation. (A) PBMCs from healthy donors were cultured for 18 h alone or in the presence of spike variants. Lipid droplets were stained with Oil red O and nuclei were stained with hematoxylin. Arrows indicate lipid droplets. Images were taken at 1000-fold magnification using 100× oil immersion objective (Olympus). Scale bars indicate 5 µm. One representative image is shown out of ten images taken from three independent experiments. (B) The number of cells positive for LDs was manually counted and the percentage was calculated based on the number of cells per field. Data are presented as mean (SD). p-values were calculated using one-way ANOVA comparing each condition with “Control”. A p-value below 0.05 was considered as significant. (C) HPMECs were cultured for 18 h alone or in the presence of spike variants. Lipid droplets were stained with Oil red O and nuclei were stained with hematoxylin. Images were taken at 1000-fold magnification using 100× oil immersion objective (Leica). Scale bars indicate 10 µm. One representative image is shown out of ten images taken from three independent experiments.
Effects of spike proteins on lipid droplet (LD) formation. (A) PBMCs from healthy donors were cultured for 18 h alone or in the presence of spike variants. Lipid droplets were stained with Oil red O and nuclei were stained with hematoxylin. Arrows indicate lipid droplets. Images were taken at 1000-fold magnification using 100× oil immersion objective (Olympus). Scale bars indicate 5 µm. One representative image is shown out of ten images taken from three independent experiments. (B) The number of cells positive for LDs was manually counted and the percentage was calculated based on the number of cells per field. Data are presented as mean (SD). p-values were calculated using one-way ANOVA comparing each condition with “Control”. A p-value below 0.05 was considered as significant. (C) HPMECs were cultured for 18 h alone or in the presence of spike variants. Lipid droplets were stained with Oil red O and nuclei were stained with hematoxylin. Images were taken at 1000-fold magnification using 100× oil immersion objective (Leica). Scale bars indicate 10 µm. One representative image is shown out of ten images taken from three independent experiments.
Lipid droplets (LDs) are cellular structures composed of a core of triacylglycerol and cholesteryl esters surrounded by phospholipids and structural proteins. While traditionally viewed as fat storage organelles, LDs have emerged as critical players in various cellular processes, including cell signaling, lipid metabolism, membrane trafficking, and inflammation. This study covered in this
COVID-19 News report, aimed to investigate whether spike proteins of SARS-CoV-2 induce LD formation in human PBMCs and pulmonary microvascular endothelial cells (HPMECs) and their potential implications.
The Investigation
Lipid Droplet Formation in Response to Spike Proteins
The research team exposed PBMCs and HPMECs to endotoxin-free recombinant variants of trimeric spike glycoproteins from SARS-CoV-2, including Alpha, Beta, Delta, and Omicron, at a concentration of 12 µg/mL. Following exposure, the cells were stained with Oil Red O to visualize LDs, and cytokine release was assessed through enzyme-linked immunosorbent assay (ELISA). Additionally, gene expression changes were examined using real-time PCR with TaqMan assays.
The results were intriguing, revealing that spike proteins induced the formation of LDs in PBMCs but not in HPMECs. Notably, the study observed differences in the size and distribution of LDs induced by various spike protein variants. For instance, "Beta" spike p
roteins appeared to have a lower effect on LD formation compared to other variants. Furthermore, "Omicron" spike proteins induced larger LDs, primarily in larger cells, as opposed to "Alpha" and "Delta" variants, which generated smaller LDs in most cells.
Impact on Lipid Metabolism Genes
To gain a deeper understanding of the molecular mechanisms involved, the researchers examined the gene expression of key players in lipid metabolism and LD formation. Spike proteins were found to decrease the expression of genes associated with lipid metabolism and LD formation, such as sterol regulatory element-binding transcription factor 1 (SREBF1), 3-Hydroxy-3-Methylglutaryl-CoA Synthase 1 (HMGCS1), low-density lipoprotein receptor (LDLR), and DNA damage-inducible transcript 3 (DDIT3), also known as C/EBP homologous protein (CHOP). Additionally, the expression of fatty acid translocase, known as CD36, was also reduced by spike proteins.
Discussion
The formation of LDs in response to various stimuli is a well-documented phenomenon in cellular biology. Inflammatory stimuli, such as lipopolysaccharide (LPS), have been shown to induce LDs in macrophages and endothelial cells, typically involving Toll-like receptor (TLR) signaling pathways. However, the mechanism behind LD accumulation triggered by SARS-CoV-2 spike proteins remains largely unexplored.
This study focused on PBMCs, a type of immune cell. Resting PBMCs typically do not contain LDs but can rapidly form LDs when activated by factors like cytokines or chemokines, either from bacterial infections or host responses. To investigate whether spike proteins trigger pro-inflammatory cytokine and chemokine production in PBMCs, the researchers conducted experiments.
Surprisingly, they found that spike proteins did not increase the production or expression of pro-inflammatory cytokines or chemokines. This suggests that LD formation in PBMCs is not solely driven by elevated cytokine or chemokine production.
A notable aspect of the study was the observation that spike-induced LD formation occurred even under conditions of complete lipid deprivation (serum-free medium). This phenomenon raises questions about the mechanisms underlying spike-induced LD formation and the potential significance of ACE2 receptor translocation in immune cells.
The study also examined the effect of spike proteins on primary human lung endothelial cells (HPMECs). In contrast to PBMCs, spike proteins did not induce LD formation in HPMECs. This observation aligns with previous findings indicating that HPMECs express very low levels of the ACE2 receptor, which is essential for SARS-CoV-2 infection. It suggests that the presence of ACE2 in spike-induced LD formation might be a contributing factor.
Furthermore, the spike-induced reduction in the expression of genes related to lipid metabolism, including HMGCS1 and LDLR1, in PBMCs supports the idea that LD formation may be a response to lipid deprivation. HMGCS1 is a key enzyme in the cholesterol biosynthesis pathway, while LDLR1 mediates cholesterol influx. The downregulation of these genes suggests a connection between lipid deprivation and LD formation.
The study also examined the expression of the PLIN2 gene, which encodes an LD surface-coating protein. PLIN2 was found to be primarily regulated at the protein level in monocytic cells, explaining why spike proteins had no significant effect on its transcript levels. Moreover, the study noted a slight reduction in the expression of CD36, a fatty acid transporter protein, in PBMCs treated with spike proteins.
Finally, the decrease in the expression of genes related to lipid metabolism and LD formation in PBMCs cultured with spike proteins under lipid-starved conditions may be associated with cellular stress responses, potentially aiding cell integrity and survival. The reduction in DDIT3 expression in response to spikes further suggests a role in cell protection and survival mechanisms.
Conclusions
In summary, the findings of this study offer important insights into the interaction between SARS-CoV-2 spike proteins and human PBMCs. The induction of lipid droplets in PBMCs by spike proteins, even under lipid-deprived conditions, raises intriguing questions about the potential role of LDs in cellular stress responses.
Moreover, the observed reduction in the expression of genes related to lipid metabolism and LD formation suggests that spike proteins may interfere with lipid metabolism pathways. This intriguing discovery warrants further investigation to unravel the complex relationship between SARS-CoV-2 spike proteins and cellular lipid homeostasis.
Understanding the mechanisms and consequences of spike-induced LD formation in immune cells is critical for advancing our knowledge of COVID-19 pathophysiology and may open doors to new therapeutic approaches. As the pandemic continues to evolve, research like this remains essential for unraveling the mysteries of the virus and its effects on the human body. Further studies are warranted to explore the specific immune cell types affected by spike-induced LD formation and the broader implications of this phenomenon in vivo.
The study findings were published in the peer reviewed journal: Microorganisms.
https://www.mdpi.com/2076-2607/11/11/2683
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
