COVID-19 News: Immune Dysfunction, Altered Immune Metabolism, Dysbiosis, Neuroinflammation And Viral Persistence Are Issues Causing Long COVID!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 25, 2024 1 year, 3 months, 15 hours, 40 minutes ago
COVID-19 News: The aftermath of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has given rise to a challenging and perplexing condition known as long COVID. A multifaceted syndrome, long COVID affects up to 10% of individuals following recovery from coronavirus disease 2019 (COVID-19). This prolonged multisystem disorder manifests with a range of debilitating symptoms, impacting daily life and raising questions about the underlying mechanisms preventing full recovery. Collaborative efforts from institutions such as the Medical University Vienna-Austria, University of Colorado School of Medicine-USA, Griffiths University-Australia, University College Cork-Ireland, University of Edinburgh-UK, and University of Zurich-Switzerland have been crucial in shedding light on the immune mechanisms that underpin the pathophysiology of long COVID.
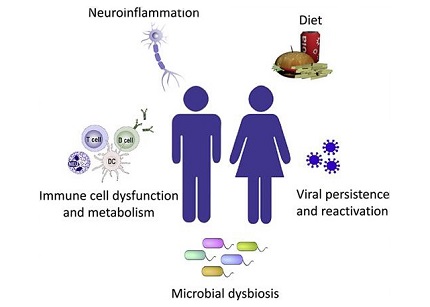 Immune mechanisms potentially impacting long COVID symptoms
Understanding Long COVID: A Multifaceted Challenge
Immune mechanisms potentially impacting long COVID symptoms
Understanding Long COVID: A Multifaceted Challenge
Long COVID, also referred to as post-acute sequelae of COVID-19 (PASC) or post-COVID condition (PCC), is a clinical diagnosis characterized by persistent symptoms emerging within three months of SARS-CoV-2 infection, persisting for at least two months without an alternative diagnosis. While the exact number of individuals affected remains uncertain, estimates from previous studies and
COVID-19 News reports suggest that at least 65 million people globally may be grappling with long COVID. Intriguingly, a significant portion of long COVID cases stems from individuals with initially mild acute illness, challenging preconceived notions about the severity of the initial infection determining the likelihood of persistent symptoms.
Immunological Mechanisms in Long COVID And Immune Dysregulation
To unravel the complexities of long COVID, it is crucial to delve into the immunological mechanisms contributing to its pathophysiology. The immune system's response to SARS-CoV-2 infection plays a pivotal role, with alterations in immune cell subsets, exhaustion markers, and dysregulation of immune mediators being observed in long COVID patients. Notably, T-cell exhaustion, inadequate immune activation during the acute phase, and altered innate immune responses may contribute to the development and persistence of long COVID symptoms.
Studies have identified specific peripheral blood cell (PBMC) subsets, including CD127lowCD8+ cells, CD4+ cells, and B cells, that are decreased or absent in long COVID patients. Elevated expression of exhaustion markers like programmed cell death protein 1 (PD1) and T-cell immunoglobulin and mucin-domain containing-3 (TIM3) suggests a potential contribution of T-cell exhaustion to symptomology.
Moreover, inadequate immune activation during the acute phase of SARS-CoV-2 infection may contribute to long COVID development. Specific antibody signatures, characterized by low IgM or low IgG3, were associated with an enhanced risk for long COVID. Gastrointestinal symptoms were linked to altered virus-specific CD8+ T cell dynamics, highlighting the importance of understandi
ng the innate immune response, which acts as the first line of defense against viral invasion.
Innate pattern recognition receptors like mannose-binding lectin (MBL) play a crucial role in neutralizing SARS-CoV-2. Low MBL levels, associated with a higher risk of SARS-CoV-2 infection, were found to be prevalent in long COVID patients experiencing severe fatigue and post-exertional malaise (PEM). This deficiency was linked to dysregulated over-production of interleukin 6 (IL-6) and tumor necrosis factor alpha, contributing to an inadequate antiviral response, viral persistence, and long-term inflammation.
Neutrophils, known for their role in protecting against SARS-CoV-2, were found to contribute to tissue injury and disease severity when in excess. Neutrophil extracellular traps (NETs) persisted at higher levels in long COVID patients compared to convalescent individuals, suggesting a potential link to pulmonary fibrosis, cardiovascular abnormalities, and neurological dysfunction.
These interactions between adaptive and innate immunity highlight the intricate nature of immunological dysregulation in the onset and progression of long COVID.
Altered Immune Metabolism
The intricate interplay between viral infection and host cell metabolism is a critical aspect of long COVID. SARS-CoV-2 infection induces changes in cellular metabolic pathways, including glycolysis and mitochondrial oxidative phosphorylation. The downregulation of oxidative phosphorylation and upregulation of glycolysis in infected cells may contribute to immune cell exhaustion and compromised antiviral responses. Furthermore, dysregulation of metabolic pathways in peripheral blood mononuclear cells (PBMCs) and altered levels of metabolites in long COVID patients point towards a potential link between immune metabolism and persistent symptoms.
The intricate interplay between viral infection and host cell metabolism is a critical aspect of long COVID. SARS-CoV-2 infection induces changes in cellular metabolic pathways, including glycolysis and mitochondrial oxidative phosphorylation (OXPHOS).
The downregulation of OXPHOS and upregulation of glycolysis in infected cells may contribute to immune cell exhaustion and compromised antiviral responses. Altered metabolic pathways in peripheral blood mononuclear cells (PBMCs) and circulating levels of metabolites in long COVID patients point towards a potential link between immune metabolism and persistent symptoms.
Viruses are obligatory parasites that depend entirely on host cells for replication. SARS-CoV-2 infection leads to increased cellular metabolic demands for virus production, resulting in changes in energy-producing processes such as glycolysis and OXPHOS.
Infection of human lung epithelium with SARS-CoV-2 leads to the release of alarmins, activation of inflammasomes, and altered antiviral protein expression. Dysregulation of immune metabolism, particularly in CD8+ T cells and monocytes, contributes to prolonged inflammation and immune dysfunction in long COVID.
Recent studies have identified a metabolic shift in long COVID patients, characterized by increased lactate production and decreased mitochondrial respiration. This altered metabolic profile may be linked to the persistent fatigue and exercise intolerance experienced by individuals with long COVID. Understanding these metabolic changes at a cellular level opens avenues for targeted therapeutic interventions aimed at restoring metabolic homeostasis and improving patient outcomes.
Viral Persistence and Reactivation
A persistent SARS-CoV-2 reservoir in host organs and the reactivation of latent viruses post-acute infection emerge as potential drivers for ongoing immune dysfunction in long COVID. The gastrointestinal tract, acting as an entry site and reservoir for SARS-CoV-2, presents the possibility of prolonged shedding and viral persistence. Additionally, the reactivation of other viruses, such as Epstein-Barr virus (EBV), may contribute to prolonged pulmonary inflammation and respiratory symptoms.
Neuroinflammation
The neurotropic nature of coronaviruses and the association of neurological complications with acute COVID-19 highlight the role of neuroinflammation in long COVID. Direct neural invasion, endothelial inflammation, and the release of pro-inflammatory cytokines contribute to brain injury and a spectrum of neurological symptoms, ranging from anosmia and ageusia to more severe conditions like stroke and autoimmune encephalitis. The intricate interplay between direct infection, endothelial inflammation, and autoimmune mechanisms underscores the complexity of neuroimmunological aspects in long COVID.
Microbiota Dysbiosis
The human microbiome, residing in mucosal surfaces and body cavities, plays a crucial role in regulating host immune responses. Long COVID is associated with persistent changes in gut bacteria and fungi, resembling patterns observed during acute COVID-19 severity. Dysbiosis, characterized by alterations in microbial diversity and the abundance of specific taxa, may contribute to prolonged immune dysregulation and inflammation in long COVID. Understanding these microbiota changes could pave the way for targeted interventions using probiotics and prebiotics to restore immune homeostasis.
Dietary Considerations in Long COVID
Malnutrition and dietary components emerge as potential influencers of long COVID outcomes. Nutrients like vitamin D, niacin, vitamins C and E, selenium, zinc, and omega-3 fatty acids play essential roles in immune function, and their deficiency may impact recovery. Moreover, overall dietary patterns, including antioxidant-rich and anti-inflammatory components, may influence outcomes in long COVID. The indirect effects of diet on the immune system, mediated through the microbiota, further emphasize the importance of a balanced and diverse diet in promoting immune health.
Beyond the Immunological Tapestry: The Social and Psychological Dimensions
While the focus has primarily been on the immunological intricacies of long COVID, it is essential to acknowledge the social and psychological dimensions of this prolonged condition. Long COVID significantly impacts mental health, with individuals grappling with uncertainty, fatigue, and the challenges of navigating daily life with persistent symptoms. Social support, mental health interventions, and a holistic approach that considers the interconnectedness of physical and mental well-being are crucial aspects of addressing the comprehensive impact of long COVID.
Conclusion - Navigating the Uncharted Waters of Long COVID
As our understanding of long COVID deepens, the intricate web of immunological, metabolic, viral, neuroinflammatory, and microbiota-related mechanisms comes into focus. Collaborative efforts from global institutions have provided invaluable insights, yet the path to effective diagnostics and treatments remains challenging. The inadequacy of current diagnostic and treatment options underscores the need for targeted clinical trials aligning experimental interventions with the diverse immunological mechanisms at play in long COVID. Moving forward, a comprehensive approach addressing the complexities of immune dysfunction, altered metabolism, dysbiosis, neuroinflammation, and viral persistence is essential to pave the way for improved outcomes and a clearer understanding of post-SARS-CoV-2 sequelae. Integrating social and psychological dimensions into the care continuum ensures a holistic approach to support individuals on their journey through the uncharted waters of long COVID.
The study findings were published in the peer reviewed journal: International Archives of Allergy and Immunology (Karger).
https://karger.com/iaa/article/doi/10.1159/000535736/893991/Immune-Mechanisms-Underpinning-Long-COVID
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
