COVID-19 News: Ohio Study Finds That SARS-CoV-2 Nsp3 Protein Triggers Cell Death And Exacerbates Amyloid Βeta42-Mediated Neurodegeneration!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 03, 2024 1 year, 3 months, 1 week, 6 days, 2 hours, 45 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has left an indelible mark on global health. Beyond the acute respiratory symptoms, mounting evidence suggests that the virus can induce long-term effects, collectively known as post-COVID-19 syndrome (PCS). A recent study conducted at the University of Dayton in Ohio and Indiana State University that is covered in this
COVID-19 News report, sheds light on a specific protein of SARS-CoV-2, Nsp3, and its role in triggering cell death, particularly in the context of neurodegenerative disorders such as Alzheimer's disease (AD).
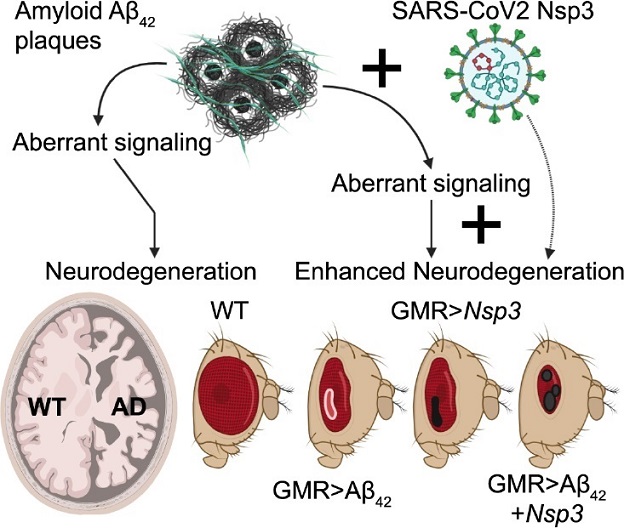 Model showing the role of Nsp3 in enhancing neurodegeneration in Alzheimer’s disease model.AD: Alzheimer’s disease; Aβ: amyloid-β; Nsp3: nonstructural protein 3; SARS-CoV2: severe acute respiratory syndrome coronavirus 2; WT: wild-type.
Understanding COVID-19 and Post-COVID-19 Syndrome
Model showing the role of Nsp3 in enhancing neurodegeneration in Alzheimer’s disease model.AD: Alzheimer’s disease; Aβ: amyloid-β; Nsp3: nonstructural protein 3; SARS-CoV2: severe acute respiratory syndrome coronavirus 2; WT: wild-type.
Understanding COVID-19 and Post-COVID-19 Syndrome
COVID-19 manifests with a spectrum of symptoms, from mild respiratory distress to severe acute respiratory syndrome (SARS), and can even extend to neurological complications such as "brain fog," cognitive dysfunction, and cardiovascular defects. The long-term effects of PCS on neurodegenerative diseases like Alzheimer's remain a largely unexplored territory. Recent studies indicate that the aging population and individuals with preexisting neurological conditions may be more susceptible to PCS, raising concerns about the potential exacerbation of conditions like AD.
Structure of SARS-CoV-2 and Cellular Interactions
SARS-CoV-2 is a pleomorphic positive-sense RNA nidovirus with helical symmetry, encoding structural proteins (N, M, S, E) and non-structural proteins (Nsp1–Nsp16) critical for its lifecycle. The virus gains entry into cells via ACE2 receptors expressed in various tissues, including neuronal cells. This broad tissue range contributes to the virus's infectivity and long-lasting impacts, including PCS. Mutations in viral proteins, especially the spike protein and the receptor binding domain, are implicated in changes in infectivity among different variants.
The Impact of SARS-CoV-2 on Cellular Processes
During infection, SARS-CoV-2 induces an upregulation of inflammatory proteins, leading to a cytokine storm with downstream effects that can result in chronic health issues, including neurodegeneration. Emerging evidence also suggests links between COVID-19 and new-onset cardiovascular and autoimmune diseases. However, the precise mechanisms through which SARS-CoV-2 proteins affect neuronal systems, particularly in individuals with preexisting neurodegenerative conditions like AD, are still underexplored.
Alzheimer's Disease and Neuroinflammation
Alzheimer's disease is a progressive, age-related neurodegenerative disorder characterized by disorientation, cognitive decline, and the accumulation of amyloid plaques (Aβ42) and neurofib
rillary tangles. Neuroinflammation, oxidative stress, and altered immune activity are known to influence AD progression, impacting cellular signaling pathways and potentially leading to cell death. Understanding the interplay between neurodegenerative disorders and SARS-CoV-2 infection is crucial for unraveling the complexities of post-COVID-19 neurological symptoms.
Utilizing Drosophila melanogaster as a Model System
To investigate the impact of individual SARS-CoV-2 proteins, the researchers employed the Drosophila melanogaster model due to its versatility, short life cycle, and the availability of molecular genetic tools. Using the Gal4/UAS system, the study focused on targeted misexpression of the Nsp3 protein in retinal neurons, allowing for in vivo testing and providing insights beyond computational predictions.
Results and Findings
The study identified Nsp3, a papain-like protease, as a key player in triggering cell death. Misexpression of Nsp3 in retinal neurons led to the generation of dark necrotic spots, increased reactive oxygen species (ROS) production, and activation of apoptosis and autophagy mechanisms, ultimately impacting tissue homeostasis. The researchers extended their investigation to a murine neuroblastoma cell line, where Nsp3 expression significantly reduced cellular metabolic activity and triggered cell death.
Moreover, in an Alzheimer's disease transgenic fly model, the study revealed that misexpression of Nsp3 exacerbated the neurodegenerative phenotype associated with high levels of cell death. The findings strongly suggest that SARS-CoV-2 utilizes the Nsp3 protein to potentiate cell death responses in a neurodegenerative disease background characterized by pre-existing neuroinflammation.
The details of the study findings are as follows:
-Nsp3 Misexpression and Neurodegeneration: The study involved targeted misexpression of the Nsp3 protein in retinal neurons using the Gal4/UAS system in Drosophila melanogaster. Strikingly, this misexpression resulted in the formation of dark necrotic spots in the retinal tissue. These spots were indicative of severe tissue damage and raised critical questions about the underlying processes triggered by the Nsp3 protein. The findings in the Drosophila model laid the foundation for further investigations into the impact of Nsp3 on cellular homeostasis.
-Reactive Oxygen Species (ROS) Production: A key aspect of the study was the assessment of reactive oxygen species (ROS) production following Nsp3 misexpression. Reactive oxygen species, including free radicals, play a crucial role in cellular signaling and homeostasis. The researchers observed a significant increase in ROS levels in response to Nsp3 misexpression, suggesting a potential link between the viral protein and oxidative stress. Elevated ROS levels are associated with various pathological conditions, including neurodegenerative disorders.
-Apoptosis and Autophagy Activation: The study further explored the cellular responses triggered by Nsp3 misexpression. It was observed that the presence of Nsp3 activated both apoptosis and autophagy mechanisms in retinal neurons. Apoptosis, or programmed cell death, is a fundamental process in multicellular organisms, while autophagy involves the degradation and recycling of cellular components. The simultaneous activation of these mechanisms indicated a profound impact of Nsp3 on the regulation of cell survival and death pathways.
-
Impact on Cellular Metabolic Activity: To extend the relevance of their findings, the researchers employed a murine neuroblastoma cell line to assess the impact of Nsp3 on cellular metabolic activity. The results demonstrated a significant reduction in metabolic activity in cells expressing Nsp3 compared to control cells. This reduction in metabolic activity is indicative of cellular distress and dysfunction, further implicating Nsp3 in detrimental cellular alterations.
-Exacerbation of Neurodegenerative Phenotype in AD Transgenic Flies: Perhaps one of the most significant findings was the investigation into the interaction between Nsp3 and pre-existing neurodegenerative conditions, particularly AD. Using a transgenic fly model of Alzheimer's disease, the study revealed that misexpression of Nsp3 exacerbated the neurodegenerative phenotype. This suggested that the presence of Nsp3 could intensify the progression of neurodegenerative disorders characterized by pre-existing neuroinflammation.
-Cell Death Signaling Pathways: While the study successfully identified Nsp3 as a key player in triggering cell death responses, the specific signaling pathways activated by Nsp3 remain an area for further exploration. Understanding the intricacies of these pathways is crucial for developing targeted therapeutic interventions to mitigate the detrimental effects of Nsp3 in the context of SARS-CoV-2 infection.
The results and findings from this study not only contribute to our understanding of the molecular underpinnings of SARS-CoV-2's impact on neurodegeneration but also open avenues for future research aimed at unraveling the complexities of virus-host interactions. As scientists continue to explore the multifaceted nature of COVID-19 and its long-term consequences, these findings may pave the way for the development of targeted treatments and interventions for individuals facing post-COVID-19 neurological complications, especially those with underlying neurodegenerative conditions.
Implications and Future Directions
The implications of these findings are far-reaching, pointing to a potential link between SARS-CoV-2 infection and the exacerbation of neurodegenerative disorders. The study underscores the need for a more comprehensive understanding of the molecular mechanisms through which viral proteins like Nsp3 impact populations with preexisting neurodegeneration.
The study's limitations include a focus on Nsp3 without exploring the impact of other SARS-CoV-2 proteins. Additionally, the specific molecular pathways through which Nsp3 activates cell death mechanisms remain to be fully elucidated. Future research endeavors could address these gaps and further investigate the impact of SARS-CoV-2 on aging populations and individuals with diverse neurodegenerative conditions.
Conclusion
In conclusion, the Ohio-Indiana study provides valuable insights into the intricate relationship between SARS-CoV-2 and neurodegenerative disorders, particularly Alzheimer's disease. By utilizing the Drosophila model, the researchers have identified Nsp3 as a significant contributor to neurodegeneration, shedding light on potential therapeutic targets for mitigating the long-term neurological effects of COVID-19.
As the world continues to grapple with the aftermath of the COVID-19 pandemic, comprehensive research endeavors like these contribute to a deeper understanding of the virus's impact on various bodily systems. Unraveling the complexities of post-COVID-19 neurological symptoms is essential for developing targeted interventions and improving the overall management of long-term health consequences associated with SARS-CoV-2 infection.
The study findings were published in the peer reviewed journal: Neural Regeneration Research.
https://journals.lww.com/nrronline/fulltext/2024/06000/sars_cov2_nsp3_protein_triggers_cell_death_and.44.aspx
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
