COVID-19 News: P21-Activated Kinase 1 (PAK1)-Mediated Cytoskeleton Rearrangement Facilitates SARS-CoV-2 Entry And ACE2 Autophagic Degradation
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 10, 2023 1 year, 6 months, 5 days, 13 hours, 31 minutes ago
COVID-19 News: The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has created unprecedented challenges for healthcare systems and economies worldwide. The rapid evolution of new viral strains, exemplified by the Omicron variant, has further complicated efforts to develop effective antiviral strategies. While vaccines have played a crucial role in reducing the severity of COVID-19, they may not fully prevent virus transmission. Direct-acting antivirals like nirmatrelvir/ritonavir and molnupiravir are effective only within the first few days of infection, leaving a gap in treatment options for later stages of the disease. This has spurred the urgent need for novel therapeutic approaches to combat COVID-19.
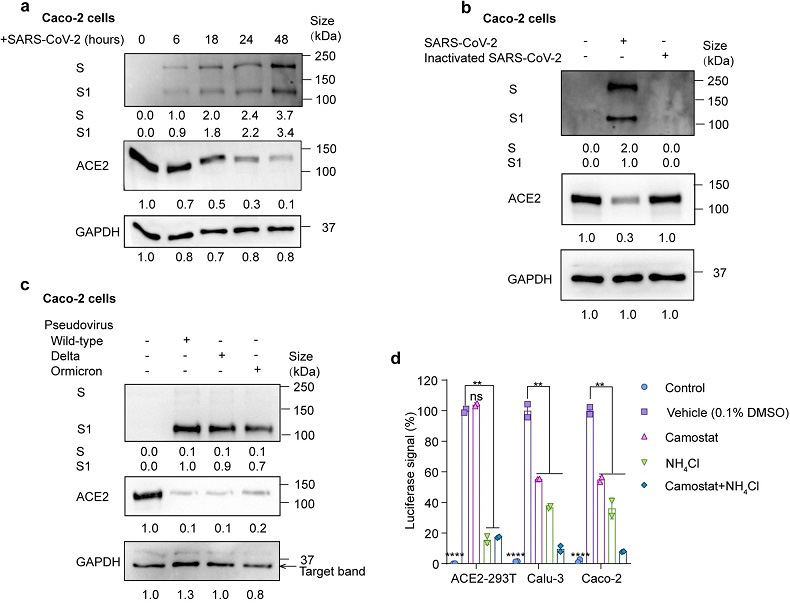 SARS-CoV-2 infection promotes ACE2 degradation. a Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 at 6, 18, 24 and 48 h post-infection (MOI = 0.01). S and S1 were detected using an anti-S1 antibody. b Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 and heat-inactivated SARS-CoV-2 at 24 h post-infection (MOI = 0.01). S and S1 were detected using an anti-S1 antibody. c Immunoblot analysis of extracts of Caco-2 cells infected by pseudotyped SARS-CoV-2 variants (Wild-type (WT), delta and omicron) at 24 h post-infection. S and S1 were detected using an anti-S1 antibody. d Luciferase signals showing entry of SARS-CoV-2 pseudovirus into three cell lines (ACE2-293T, Calu-3, Caco-2) with vehicle (H2O), TMPRSS2 inhibitor camostat (50 μM) or endocytosis inhibitor NH4Cl (2 mM) for 48 h. Uninfected cells were included as negative control. Viral entry efficiency was calculated as the percentages of luciferase signal with inhibitors/luciferase signal with vehicle (H2O). Error bars indicate SEM. P-values were calculated by Tukey’s multiple comparison test. **P < 0.01; ****P < 0.0001; ns: not significant. e Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 pseudovirus for 24 h with the treatment of camostat or NH4Cl at the indicated concentration
SARS-CoV-2 infection promotes ACE2 degradation. a Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 at 6, 18, 24 and 48 h post-infection (MOI = 0.01). S and S1 were detected using an anti-S1 antibody. b Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 and heat-inactivated SARS-CoV-2 at 24 h post-infection (MOI = 0.01). S and S1 were detected using an anti-S1 antibody. c Immunoblot analysis of extracts of Caco-2 cells infected by pseudotyped SARS-CoV-2 variants (Wild-type (WT), delta and omicron) at 24 h post-infection. S and S1 were detected using an anti-S1 antibody. d Luciferase signals showing entry of SARS-CoV-2 pseudovirus into three cell lines (ACE2-293T, Calu-3, Caco-2) with vehicle (H2O), TMPRSS2 inhibitor camostat (50 μM) or endocytosis inhibitor NH4Cl (2 mM) for 48 h. Uninfected cells were included as negative control. Viral entry efficiency was calculated as the percentages of luciferase signal with inhibitors/luciferase signal with vehicle (H2O). Error bars indicate SEM. P-values were calculated by Tukey’s multiple comparison test. **P < 0.01; ****P < 0.0001; ns: not significant. e Immunoblot analysis of extracts of Caco-2 cells infected by SARS-CoV-2 pseudovirus for 24 h with the treatment of camostat or NH4Cl at the indicated concentration
Coronaviruses, including SARS-CoV-2, are single-stranded RNA viruses that utilize various entry pathways to infect host cells. Understanding these mechanisms is essential for developing effective treatments. SARS-CoV-2 can enter host cells via endosomal and non-endosomal pathways, primarily involving cathepsin-mediated endocytosis and TMPRSS2-mediated fusion, respectively. The spike protein on the viral surface plays a pivotal role in facilitating viral attachment and entry into host cells. This glycoprotein can be cleaved into S1 and S2 subunits by various enzymes, including TMPRSS2, furin, and lysosomal cathepsins, allowing the virus to bind to host cells and mediate membrane fusion.
The host receptor angiotensin-converting enzyme 2 (ACE2) is crucial for SARS-CoV-2 entry into host cells. ACE2 normally catalyzes angiotensin II (AngII) into angiotensin 1-7, thereby regulating the renin-angiotensin system (RAS) to prevent overactivation. However, SARS-CoV-2 infection has been shown to lead to ACE2 degradation, impacting its role in maintaining RAS balance. While this degradation process has been observed, the underlyin
g molecular mechanisms and its implications for SARS-CoV-2 infection have remained unclear.
This
COVID-19 News report explores recent research findings by scientists from Guangzhou Medical University-China, Southern Medical University, Guangdong-China, The University of Hong Kong, Pokfulam-China and The Third Affiliated Hospital of Zhengzhou University-China that shed light on the interplay between SARS-CoV-2, ACE2, and the cellular processes involved in viral entry and infection.
Specifically, the study delves into the role of P21-activated kinase 1 (PAK1)-mediated cytoskeleton rearrangement in facilitating viral entry and the potential of a pan-PAK inhibitor, FRAX-486, as a promising therapeutic agent against SARS-CoV-2.
SARS-CoV-2 Entry Promotes ACE2 Degradation
One of the key findings of this study is that SARS-CoV-2 entry into host cells triggers the degradation of ACE2. ACE2, when bound to the viral spike protein, forms a complex that is subsequently endocytosed via clathrin-mediated endocytosis. The involvement of clathrin, a protein that plays a pivotal role in vesicle formation and transport within cells, suggests a carefully orchestrated process. PAK1, a signaling molecule known for its role in cytoskeleton rearrangement, is also implicated in this process. As the ACE2-spike complex is internalized, PAK1-mediated cytoskeleton rearrangement appears to facilitate its degradation through autophagy.
Autophagy-Lysosome Pathway and Viral Entry
Autophagy is a cellular process responsible for degrading damaged organelles, proteins, and other cellular components in lysosomes. In the context of SARS-CoV-2 infection, autophagy plays a vital role in degrading the ACE2-spike complex, thereby facilitating the release of the viral genome. This mechanism aids the virus in successfully entering the host cell, highlighting the intricate interplay between viral strategies and host cellular processes.
Cytoskeleton Rearrangement: The Role of PAK1
PAK1, a critical player in cell signaling pathways, appears to be instrumental in facilitating SARS-CoV-2 entry. By rearranging the cellular cytoskeleton, PAK1 contributes to the endocytosis of the ACE2-spike complex. This process is crucial for viral entry and infection. Furthermore, PAK1 inhibition emerges as a potential strategy to restore ACE2 expression and, subsequently, suppress SARS-CoV-2 infection.
Potential Therapeutic Implications of FRAX-486
The pan-PAK inhibitor FRAX-486 emerges as a promising candidate for the treatment of COVID-19. In vitro experiments demonstrate its potent and broad-spectrum activity against various SARS-CoV-2 strains, including the challenging Omicron variant. Additionally, in vivo studies using a SARS-CoV-2-infected hamster model show that FRAX-486 effectively reduces viral load and alleviates pulmonary inflammation, offering hope for a novel therapeutic approach.
The Significance of ACE2 Restoration
Restoring ACE2 surface expression presents a compelling strategy for mitigating the severity of COVID-19. By preventing the overactivation of the renin-angiotensin system (RAS), ACE2 can help protect against lung injury caused by respiratory viruses. This becomes particularly relevant for vulnerable populations, such as the elderly and individuals with cardiovascular diseases, diabetes, or hypertension, who are at higher risk of severe COVID-19. ACE2 downregulation in these conditions may contribute to disease progression, making ACE2 restoration an attractive therapeutic target.
Limitations and Future Directions
While this study provides valuable insights into the mechanisms of SARS-CoV-2 entry and ACE2 degradation, certain limitations should be acknowledged. The exact role of ADAM17, a protein associated with ACE2 shedding in SARS-CoV infection, remains to be fully elucidated in the context of SARS-CoV-2 infection. Further research is needed to better understand the interplay between PAK1, viral entry, and host cell responses, particularly in different cell types and tissues.
Conclusion
In conclusion, this research illuminates the intricate interactions between SARS-CoV-2, ACE2, and host cell processes involved in viral entry and infection. PAK1-mediated cytoskeleton rearrangement emerges as a critical facilitator of viral entry, and the pan-PAK inhibitor FRAX-486 shows promise as a therapeutic agent against SARS-CoV-2. Restoring ACE2 surface expression may not only ameliorate disease severity but also restrict viral propagation. As the world grapples with the ongoing challenges posed by COVID-19 and its variants, these findings open new avenues for the development of innovative treatments and therapeutic strategies to combat the virus. Further research and clinical trials are warranted to explore the full potential of PAK1 inhibition and ACE2 restoration in the fight against COVID-19.
The study findings were published in the peer reviewed journal: Signal Transduction And Targeted Therapy (Nature).
https://www.nature.com/articles/s41392-023-01631-0
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
