COVID-19 News: Pittsburg Study Shows That SARS-CoV-2 ORF7b Protein Induces Lung Injury Via c-Myc Mediated Apoptosis And Ferroptosis
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 20, 2024 1 year, 2 months, 3 weeks, 4 days, 9 hours, 31 minutes ago
COVID-19 News: In the relentless battle against the global COVID-19 pandemic, scientists and researchers around the world are continuously striving to uncover the intricacies of the SARS-CoV-2 virus to develop effective treatments and strategies. One such breakthrough comes from the University of Pittsburgh, USA, where a comprehensive study sheds light on the role of the open reading frame 7b (Orf7b) protein in orchestrating lung injury through c-Myc mediated apoptosis and ferroptosis. This groundbreaking research covered in this
COVID-19 News report not only deepens our understanding of the molecular mechanisms behind SARS-CoV-2-induced lung damage but also identifies potential therapeutic targets for mitigating the severity of COVID-19.
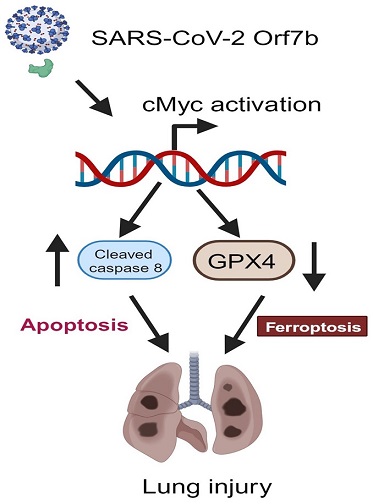 Scheme for the Orf7b-induced cell death pathways, apoptosis, and ferroptosis, in lung epithelial cell and mouse models. The SARS-CoV-2 accessory protein Orf7b induces cell death pathways in both extrinsic apoptosis and ferroptosis. Orf7b increases c-Myc at the protein level to modulate apoptosis and ferroptosis. Increased apoptosis and ferroptosis thus promote acute lung injury.
The Complex Landscape of SARS-CoV-2 Infection
Scheme for the Orf7b-induced cell death pathways, apoptosis, and ferroptosis, in lung epithelial cell and mouse models. The SARS-CoV-2 accessory protein Orf7b induces cell death pathways in both extrinsic apoptosis and ferroptosis. Orf7b increases c-Myc at the protein level to modulate apoptosis and ferroptosis. Increased apoptosis and ferroptosis thus promote acute lung injury.
The Complex Landscape of SARS-CoV-2 Infection
SARS-CoV-2, a member of the coronaviridae family, has proven to be a formidable adversary, causing a global pandemic with millions of infections and deaths. Understanding the molecular intricacies of SARS-CoV-2 infection is crucial for devising effective therapeutic interventions. The virus enters host cells primarily through the interaction of its spike protein with angiotensin-converting enzyme 2 (ACE2), expressed prominently in ciliated airway epithelia and alveolar type II cells. Despite its multi-organ impact, acute lung injury and respiratory distress syndrome are central to the severe manifestations of COVID-19.
Cell Death Pathways in COVID-19 Pathogenesis
The pathophysiology of COVID-19 is marked by extensive tissue damage, reflected in elevated levels of lactate dehydrogenase (LDH) and Troponin I, indicating cellular demise. Autopsies of COVID-19 lungs reveal widespread epithelial and endothelial cell death. The study at the University of Pittsburgh identifies various cell death pathways activated by SARS-CoV-2, including accidental (necrotic) and regulated cell death. Of particular interest are apoptotic and non-apoptotic regulated cell death types, such as ferroptosis, characterized by iron-dependent lipid peroxidation.
Orf7b Emerges as a Key Player
Among the myriad viral factors, Orf7b emerges as a pivotal contributor to lung epithelial cell death. Through meticulous experiments, the researchers demonstrate that overexpression of Orf7b induces both apoptotic and ferroptotic cell death, unraveling a previously undisclosed facet of SARS-CoV-2 pathogenesis. The concentration-dependent and time-dependent nature of Orf7b-induced cytotoxicity underscores its importance as a viral factor in promoting lung epithelial cell death.
Total RNA seq Analysis Unravels Molecular Pathways
The researchers employed total RNA sequencing to d
elve into the molecular intricacies triggered by Orf7b. Ingenuity Pathway Analysis (IPA) predicted the involvement of death receptor signaling, ferroptosis, apoptosis, and c-Myc-mediated apoptosis signaling. Functional enrichment analysis highlighted c-Myc as a key transcription regulator, establishing a link between Orf7b-induced cell death and the regulation of essential pathways by c-Myc.
Orf7b Induces Both Apoptosis and Ferroptosis - Experimental Validation
To validate the predictions from total RNA sequencing, in vitro experiments using lung epithelial cells were conducted. The results confirmed that Orf7b overexpression led to the upregulation of cleaved caspase 8, a marker of extrinsic apoptotic cell death. Additionally, the ferroptosis regulator GPX4 was downregulated, accompanied by elevated levels of malondialdehyde (MDA) and reactive oxygen species (ROS). These findings provide conclusive evidence that Orf7b induces cell death through both apoptotic and ferroptotic pathways.
c-Myc Emerges as the Orchestrator
Consistent with the RNA-seq results, c-Myc was identified as the central regulator of Orf7b-induced apoptosis and ferroptosis. Orf7b overexpression increased c-Myc expression, and depletion of c-Myc prevented Orf7b-induced cell death, as validated by the LDH assay and reduced intracellular ROS levels. This discovery establishes c-Myc as a key player in the intricate dance of molecular events orchestrated by Orf7b, further underscoring the importance of this transcription regulator in SARS-CoV-2 pathogenesis.
Orf7b's In Vivo Impact: Mouse Model Validation
To validate the in vivo relevance of their findings, the researchers turned to a mouse model. Intranasal administration of adenoviral-associated serotype 9 vectors was utilized to overexpress Orf7b in mouse airways. The results were striking, as Orf7b overexpression induced severe lung injury, characterized by inflammatory cell infiltration and increased bronchoalveolar lavage fluid (BALF) protein content. Importantly, c-Myc depletion mitigated Orf7b-induced lung injury, providing tangible evidence of the crucial role of c-Myc in the pathogenic effects of Orf7b.
Unveiling the Structural Nuances of Orf7b
The study delves into the structural characteristics of Orf7b, providing valuable insights into its molecular makeup. Orf7b, with a predicted molecular weight of 5.1 kDa, consists of 43 amino acid residues enriched in leucine (25.58%) and phenylalanine (13.95%). Sequence analysis reveals an 88% sequence identity with SARS Orf7b, showcasing the conservation of key structural elements. The presence of a fully conserved transmembrane domain, two predicted helices, one helix–helix interaction domain, one beta turn, and one gamma turn without channel formation adds another layer of complexity to the functionality of Orf7b.
Connecting Orf7b to Immune Response and Host Pathways
Orf7b, along with other viral factors, is reported to suppress Type-1 interferon (IFN) responses. The study highlights the multifaceted role of Orf7b in disrupting several important cellular networks and deregulating key host pathways, such as metabolism, cell adhesion, and immune response. This novel insight opens avenues for future investigations into the intricate interplay between Orf7b and host cellular mechanisms.
Ferroptosis in COVID-19 Multiorgan Damage - A Modest Link
While previous data on ferroptosis in the context of COVID-19 multiorgan damage are modest, the study at the University of Pittsburgh marks a significant stride. Ferroptosis, a recently identified type of cell death involving iron-dependent generation of hydroperoxy phospholipids, has been reported in sinoatrial pacemaker cells, placenta, and endothelial cells. The study is one of the first to identify SARS-CoV-2-induced ferroptosis in lung epithelial cells and in an in vivo lung model. The activation of both apoptosis and ferroptosis by Orf7b adds a layer of complexity to our understanding of COVID-19 pathogenesis.
Conclusion
In conclusion, the research from the University of Pittsburgh represents a monumental step forward in unraveling the molecular choreography of SARS-CoV-2-induced lung injury. Orf7b emerges as a central player, orchestrating both apoptotic and ferroptotic cell death in lung epithelial cells. The intricate interplay between Orf7b and the transcription regulator c-Myc unveils a novel layer of complexity in COVID-19 pathogenesis. The findings not only deepen our understanding of the virus's impact on lung cells but also open new avenues for therapeutic interventions. Targeting Orf7b or modulating c-Myc activity could prove instrumental in mitigating the severity of COVID-19, offering hope in the ongoing battle against the pandemic. As the scientific community continues to decode the mysteries of SARS-CoV-2, this research stands as a beacon, guiding future endeavors towards a more comprehensive understanding of COVID-19 pathophysiology.
The study findings were published in the peer reviewed International Journal of Molecular Sciences.
https://www.mdpi.com/1422-0067/25/2/1157
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
