COVID-19 News: SARS-CoV2 Nsp3 Triggers Cell Death And Exacerbates Amyloid β42-Mediated Neurodegeneration
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 28, 2024 1 year, 2 months, 1 week, 7 hours, 29 minutes ago
COVID-19 News: The ongoing COVID-19 pandemic has led to an array of symptoms, ranging from acute respiratory distress to lingering neurological effects. Among the latter, cognitive dysfunction and post-COVID-19 syndrome (PCS) have emerged as areas requiring further investigation. This study covered in this
COVID-19 News report, conducted jointly by the University of Dayton, Ohio, and Indiana State University, delves into the impact of the SARS-CoV2 virus on neurodegenerative disorders, specifically Alzheimer's disease (AD). By focusing on the nonstructural protein 3 (Nsp3), the research sheds light on the intricate relationship between SARS-CoV2 infection and age-related progressive neurodegeneration.
 SARS-CoV2 Nsp3 Triggers Cell Death And Exacerbates Amyloid β42-Mediated Neurodegeneration
SARS-CoV2 and Neurological Impact
SARS-CoV2 Nsp3 Triggers Cell Death And Exacerbates Amyloid β42-Mediated Neurodegeneration
SARS-CoV2 and Neurological Impact
The SARS-CoV2 virus exhibits diverse symptomatic presentations, both acute and chronic. While acute symptoms primarily involve respiratory distress, severe cases may lead to complications such as acute respiratory distress syndrome (ARDS). However, the lingering effects of COVID-19, known as PCS, have raised concerns, especially regarding its impact on individuals with preexisting neurological conditions like AD. The virus's ability to infect a variety of tissues, including neuronal cells, makes it a potential contributor to neurodegenerative disorders.
Structural and Nonstructural Proteins of SARS-CoV2
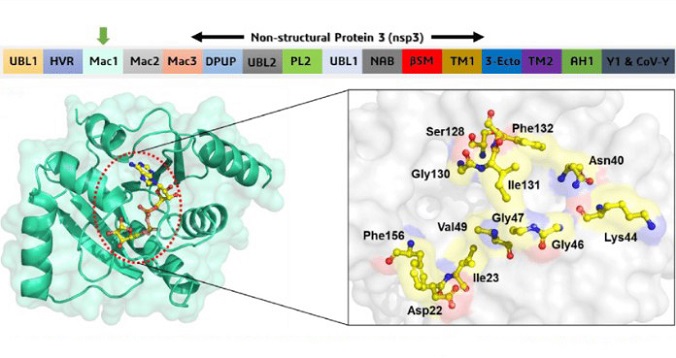
The SARS-CoV2 virus, a pleomorphic positive-sense RNA nidovirus, encodes several structural and nonstructural proteins. These proteins play crucial roles in viral replication and pathogenesis. Of particular interest in this study is the nonstructural protein 3 (Nsp3), a papain-like protease involved in the release of other nonstructural proteins essential for viral replication.
Using Drosophila Melanogaster as a Model System
To unravel the impact of SARS-CoV2 proteins, the researchers employed the Drosophila melanogaster model due to its versatility and genetic tractability. This model system allowed for in vivo testing of individual SARS-CoV2 proteins, including Nsp3, in the retinal neurons of the fruit fly's eye. The results demonstrated that misexpression of Nsp3 triggered a range of cellular responses, leading to neurodegenerative phenotypes.
Nsp3-Induced Cell Death in Drosophila and Neuro-2a Cells
The study found that targeted misexpression of Nsp3 in Drosophila retinal neurons resulted in dark necrotic spots and the activation of apoptotic and autophagic mechanisms. This was further corroborated in murine neuroblastoma cells (Neuro-2a), where transient expression of Nsp3 led to a significant reduction in metabolic activity and increased cell death. These findings emphasize the pathogenic potential of Nsp3 in inducing cell death responses.
Linking Nsp3 to Alzheimer's Disease (AD)
Given the impact of Nsp3 in a normal background, the rese
archers explored its effects in a neurodegenerative context, specifically using an AD transgenic fly model (GMR>Aβ42). The results indicated that Nsp3 misexpression exacerbated the neurodegenerative phenotype in the AD background, suggesting that SARS-CoV2 may utilize Nsp3 to potentiate cell death responses in individuals with preexisting neuroinflammation and neurodegeneration.
Long-Term Impact and Progression of Neurodegeneration
The study extended its investigation into the long-term effects of Nsp3 misexpression by utilizing pupal retina models and aging adult flies. The results demonstrated a progressive necrosis phenotype, with adult flies exhibiting depigmentation, necrotic spots, and worsening neurodegeneration over time. This observation is particularly relevant in the context of PCS, where prolonged exposure to Nsp3 may contribute to persistent symptoms.
Synergistic Effects of Nsp3 and Aβ42 in Neurodegeneration
To understand the interplay between SARS-CoV2 proteins and preexisting neurodegenerative conditions, the researchers co-expressed Nsp3 and Aβ42 in the Drosophila model. The combined effect led to a significant increase in cell death markers, suggesting a potential synergistic or additive impact on neurodegeneration. This finding underscores the intricate relationship between viral proteins and existing neurodegenerative processes.
Implications for Therapeutic Targeting
The identification of Nsp3 as a determinant of neurodegenerative phenotypes opens avenues for therapeutic targeting. Given its role in the replication/transcription complex of SARS-CoV2, Nsp3 becomes a potential therapeutic target for developing anti-SARS-CoV2 drugs. This study suggests that inhibiting Nsp3 activity could mitigate the neurodegenerative effects observed in the context of COVID-19.
Limitations and Future Directions
While this study provides valuable insights, certain limitations should be considered. The focus on Nsp3 as a singular protein may not fully capture the complexity of SARS-CoV2-induced neurodegeneration. Future studies could explore the impact of other viral proteins on neurodegenerative processes. Additionally, the study primarily addresses the effects in the context of AD, leaving room for investigations into other neurodegenerative diseases.
Conclusion
In conclusion, this comprehensive study illuminates the intricate link between the SARS-CoV2 Nsp3 protein and neurodegeneration using the Drosophila model and cell culture systems. The findings underscore the potential of SARS-CoV2 to exacerbate neurodegenerative phenotypes, especially in individuals with preexisting conditions. The identification of Nsp3 as a key player opens avenues for targeted therapeutic interventions, offering hope for mitigating the long-term neurological effects associated with COVID-19. As the scientific community continues to unravel the complexities of SARS-CoV2, studies like these pave the way for a deeper understanding of the virus's impact on neurological health.
The study findings were published in the peer reviewed journal: Neural Regeneration Research.
https://journals.lww.com/nrronline/fulltext/2024/06000/sars_cov2_nsp3_protein_triggers_cell_death_and.44.aspx
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-news-mutations-in-loop-2-of-sars-cov-2-macrodomain-2-in-nsp3-can-enhance-adp-ribose-binding-and-lead-to-more-virulent-strains
https://www.thailandmedical.news/news/covid-19-news-german-study-uncovers-that-sars-cov-2-nsp3-and-nsp4-are-important-constituents-of-a-pore-spanning-replication-organelle
https://www.thailandmedical.news/news/covid-19-news-sars-cov-2-nsp3-hijacks-fragile-x-mental-retardation-proteins-for-efficient-infection-while-modulating-stress-granule-function
https://www.thailandmedical.news/news/breaking-covid-19-news-study-discovers-a-crucial-enzyme-in-sars-cov-2-nsp3-protein-the-macro1-domain-that-plays-a-role-in-innate-immunity
https://www.thailandmedical.news/news/covid-19-news-japanese-researchers-warn-that-the-usage-of-inhaled-ciclesonide-in-driving-sars-cov-2-nsp3-and-nsp4-mutations
https://www.thailandmedical.news/news/french-researchers-uncover-that-sars-cov-2-nsp3-protein-interacts-with-human-host-rna-g-quadruplexes,-contributing-to-virulence
https://www.thailandmedical.news/news/french-researchers-unveil-critical-insights-into-sars-cov-2-s-de-marylation-activity-through-macro1-domain
