COVID-19 News: Scientists Discover New Strategy To Inhibit SARS-CoV-2 Infection By Exploiting Host Protein PRPF19 To Degrade ORF6 Via Ubiquitination!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 26, 2024 1 year, 3 months, 7 hours, 8 minutes ago
COVID-19 News: The ongoing COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spurred relentless efforts to understand the virus-host interactions and develop effective therapeutic strategies. A recent breakthrough in the battle against the virus comes from the research conducted at the Institute of Virology and AIDS Research, The First Hospital of Jilin University, Changchun, Jilin-China, and the Chinese Academy of Medical Sciences, Changchun, Jilin-China and is covered in this
COVID-19 News report. Their study focuses on the host protein PRPF19 and its remarkable role in inhibiting SARS-CoV-2 infection by orchestrating the ubiquitin-dependent degradation of the viral accessory protein ORF6.
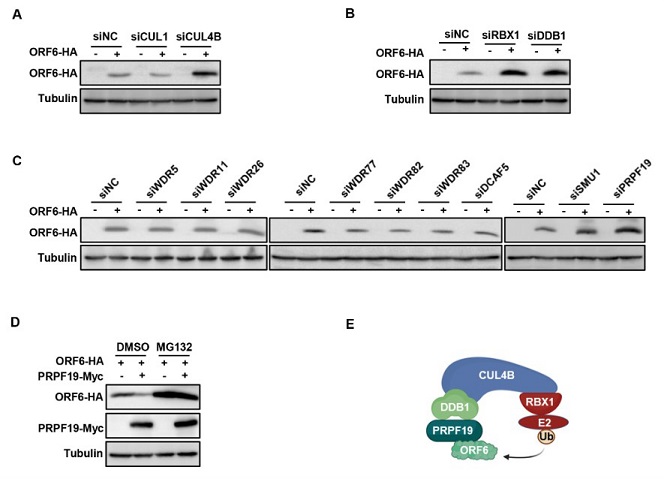 CRL4BPRPF19 E3 ligase targets ORF6 for degradation. (A–C) Knockdown of CUL4B, DDB1, RBX1, or PRPF19 increased ORF6 stability. Based on mass spectrum analysis results, CUL1, CUL4B, DDBI, RBXI, or the substrate recognition receptors (WDR5, WDR11, WDR26, WDR77, WDR82, WDR83, DCAF5, SMU1, PRPF19) were knocked down separately in HEK293T by siRNA, then the knockdown cells were transfected with ORF6-HA for 48 h. The cell lysates were analyzed by IB. (D) PRPF19 overexpression in vivo increased ORF6 degradation. ORF6 was cotransfected with a negative control vector or PRPF19 into HEK293T cells, and then, the cells were treated with or without 10 µM MG132 for 12 h prior to harvest. The cell lysates were analyzed by IB. (E) Schematic of CRL4BPRPF19-mediated ORF6 degradation. CUL4B formed a conserved E3 ligase complex with the RING-box protein RBX1, the adaptor protein DDB1, and the substrate recognition receptor PRPF19.
The Genome of SARS-CoV-2 and Host Immune Response
CRL4BPRPF19 E3 ligase targets ORF6 for degradation. (A–C) Knockdown of CUL4B, DDB1, RBX1, or PRPF19 increased ORF6 stability. Based on mass spectrum analysis results, CUL1, CUL4B, DDBI, RBXI, or the substrate recognition receptors (WDR5, WDR11, WDR26, WDR77, WDR82, WDR83, DCAF5, SMU1, PRPF19) were knocked down separately in HEK293T by siRNA, then the knockdown cells were transfected with ORF6-HA for 48 h. The cell lysates were analyzed by IB. (D) PRPF19 overexpression in vivo increased ORF6 degradation. ORF6 was cotransfected with a negative control vector or PRPF19 into HEK293T cells, and then, the cells were treated with or without 10 µM MG132 for 12 h prior to harvest. The cell lysates were analyzed by IB. (E) Schematic of CRL4BPRPF19-mediated ORF6 degradation. CUL4B formed a conserved E3 ligase complex with the RING-box protein RBX1, the adaptor protein DDB1, and the substrate recognition receptor PRPF19.
The Genome of SARS-CoV-2 and Host Immune Response
SARS-CoV-2, with a genome approximately 29.9 kb in size, encodes various proteins, including nonstructural and structural proteins, as well as accessory proteins such as ORF6. The host's innate immune system acts as the primary defense against viral infections. However, SARS-CoV-2 has evolved mechanisms to suppress interferon (IFN) production, a crucial aspect of the host's antiviral response. Among the potent IFN antagonists, ORF6 plays a pivotal role in evading the host immune system by inhibiting IFN responses. ORF6 achieves this by interfering with nuclear translocation and export processes, hindering the expression of IFN-stimulated genes.
Ubiquitin-Proteasome Pathway in Viral Pathogenesis
The ubiquitin-proteasome pathway, a fundamental cellular process, is a powerful tool employed by host cells to defend against viral infections. Some cellular proteins act as restriction factors, limiting viral infection through ubiquitin-dependent degradation. In this study, the researchers identified the CUL4B-DDB1-PRPF19 E3 Ubiquitin Ligase Complex as a mediator of proteasomal degradation of ORF6, thereby suppressing SARS-CoV-2 replication.
Unraveling the Mechanism - PRPF19 as a Key Player
The researchers employed a systematic approach to uncover the molecular details of how PRPF19 inhibits SARS-CoV-2 infection. Their investig
ation revealed that PRPF19 interacts with CUL4B, DDB1, and RBX1 to form a CRL4B-based E3 ligase complex. This complex, in turn, catalyzes the ubiquitination and subsequent degradation of ORF6. Notably, overexpression of PRPF19 was found to promote the degradation of ORF6, leading to the alleviation of IFN inhibition and inhibition of SARS-CoV-2 replication.
Specificity of PRPF19 in ORF6 Degradation
The study delved into the specificity of PRPF19 in targeting ORF6 for degradation. Mass spectrometry data revealed that PRPF19 interacts with several substrate recognition receptors, but only the knockdown of PRPF19 increased the stability of ORF6. Importantly, overexpression of PRPF19 specifically affected ORF6 stability without impacting the stability of other viral proteins, indicating the specific role of PRPF19 in ORF6 degradation.
PRPF19 Interaction and Ubiquitination of ORF6
The researchers confirmed the interaction between PRPF19 and ORF6 through co-immunoprecipitation experiments. Fluorescence resonance energy transfer (FRET) analysis further supported the direct interaction between PRPF19 and ORF6. The ubiquitination of ORF6 was then investigated, revealing that PRPF19 catalyzes K48-linked ubiquitination of ORF6. This finding provided insights into the specific type of ubiquitin chain responsible for ORF6 degradation.
Antagonizing ORF6-Mediated IFN Signaling Inhibition
Given ORF6's role as a key IFN antagonist, the study explored the impact of PRPF19 on ORF6-mediated IFN signaling inhibition. Overexpression of PRPF19 was shown to induce the degradation of ORF6, leading to the release of IFN-stimulated response element (ISRE) and the loss of IFN-antagonizing effects. Moreover, PRPF19 enhanced the mRNA levels of IFN-α, IFN-β, and IFN-stimulated genes, further underscoring its role in antagonizing ORF6-mediated IFN signaling inhibition.
PRPF19's Antiviral Role in SARS-CoV-2 Replication
To assess the broader implications of PRPF19 in SARS-CoV-2 replication, the researchers examined its impact on viral protein expression and replication in cellular and animal models. Knockdown of PRPF19 increased the levels of viral proteins, indicating enhanced SARS-CoV-2 replication. Conversely, overexpression of PRPF19 restricted viral RNA replication and protein expression. These findings established PRPF19 as a crucial factor in inhibiting SARS-CoV-2 replication.
Etoposide-Mediated Enhancement of CUL4B Activity
The researchers explored the regulatory role of neddylation, a post-translational modification, on CUL4B activity. Etoposide, a DNA damage inducer, was identified as a potential activator of CUL4B neddylation. Treatment with etoposide was found to reduce cellular protein levels of ORF6, promoting its ubiquitination and subsequent degradation. Importantly, etoposide exhibited antiviral effects by reducing SARS-CoV-2 replication in cellular and animal models.
Etoposide as a Potential Therapeutic Candidate
The study extended its findings into a mouse infection model, where etoposide treatment significantly reduced viral replication, lung lesions, and cytokine production. The observed antiviral effects of etoposide were dependent on the presence of PRPF19, further establishing the CRL4B-PRPF19 axis as a potential target for therapeutic intervention.
Conclusion
In conclusion, the research findings shed light on the intricate mechanisms by which the host protein PRPF19 inhibits SARS-CoV-2 infection. The CUL4B-DDB1-PRPF19 E3 Ubiquitin Ligase Complex emerges as a key player in orchestrating the ubiquitin-dependent degradation of ORF6, thereby suppressing viral replication. The study not only deepens our understanding of the molecular dynamics underlying SARS-CoV-2 pathogenesis but also proposes potential therapeutic strategies, with etoposide emerging as a promising candidate for further investigation. As the global scientific and medical communities continue their fight against the COVID-19 pandemic, this research provides valuable insights and avenues for the development of targeted interventions.
The study findings were published in the peer reviewed journal: mBio.
https://journals.asm.org/doi/10.1128/mbio.03071-23
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
