COVID-19 News: Study Explores A Novel SARS-CoV-2 Helicase, NSP13 - Potential Breakthrough In Anti-COVID-19 Drug Development
Thailand Medical News Team Aug 18, 2023 2 years, 5 months, 2 weeks, 3 days, 4 hours, 17 minutes ago
COVID-19 News: The global battle against the COVID-19 pandemic has brought scientific innovation to the forefront, with researchers tirelessly seeking novel approaches to combat the SARS-CoV-2 virus. Recent groundbreaking research has illuminated the potential of a previously underestimated player in the viral replication process - nonstructural protein 13 (nsp13), a helicase. Employing a multidisciplinary approach, scientists have unraveled the intricate mechanisms of nsp13 and its implications for the development of cutting-edge antiviral drugs.
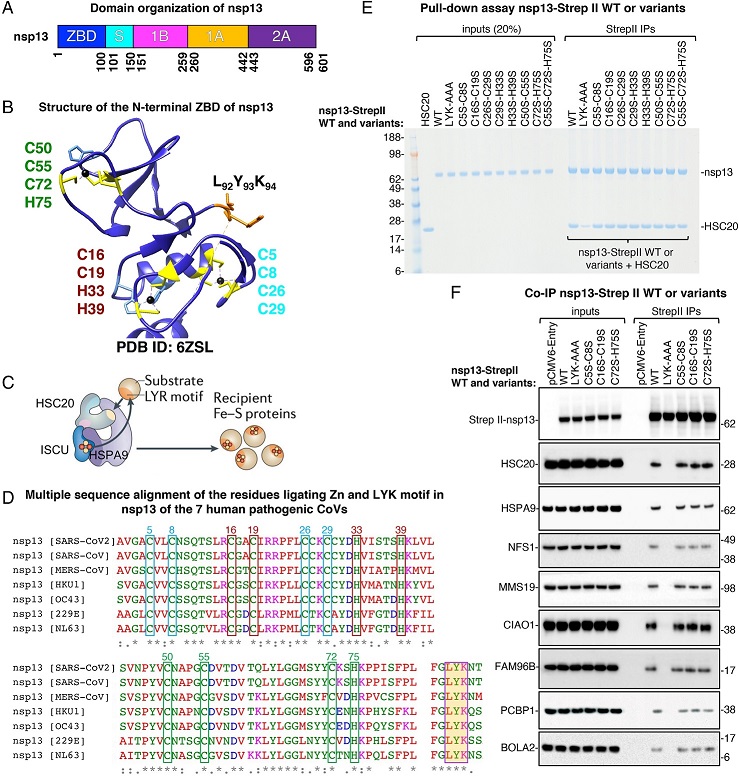 Fe–S cluster incorporation into nsp13 occurs through its interactions with components of the Fe–S biogenesis machinery. (A) Domain organization of nsp13. The ZBD is shown in dark blue, followed by a “stalk” region (light blue), a β-barrel, 1B, in magenta, and two helicase core domains RecA1 (1A) and RecA2 (A2) (19). (B) Structure of the N-terminal zinc-binding domain (ZBD) of nsp13 showing the amino acid residues ligating zinc in the available crystal structure (PDB ID: 6ZSL) (20). A completely conserved LYR-like motif (LYK) in the seven human pathogenic coronaviruses is highlighted in yellow. (C) Proposed model of the chaperone/cochaperone-mediated transfer of nascent Fe–S clusters, assembled on the main scaffold protein, ISCU, through the direct binding of the cochaperone HSC20 to LYR motifs present in recipient apoproteins. ATP hydrolysis by the HSC20-cognate chaperone, HSPA9, is proposed to facilitate cluster transfer to recipient proteins, while concomitantly driving folding of the recipient protein into its final conformation. (D) Multiple sequence alignment of the residues ligating zinc and of the conserved LYR-like motif in nsp13 of the seven human pathogenic coronaviruses. Boxes in the same color identify the amino acid residues in each individual metal-binding site. (E) Representative Coomassie blue staining of pull-down assays performed with purified proteins. Purified nsp13-Strep II (0.25 μg) or the variants wherein either the LYK motif or the cysteine and histidine residues coordinating zinc in the available structures were replaced by alanines or serines, respectively, as indicated, were combined with 0.25 μg of HSC20. Immunoprecipitations (IPs) were performed with Strep II antibody to immunocapture nsp13 proteins. The presence of HSC20 (a.k.a. HSCB) in the eluates after IPs of nsp13 proteins was analyzed by SDS-polyacrylamide gel electrophoresis and Coomassie staining. Aliquots corresponding to 20% of the inputs were run on the gel for comparison (n = 4 biological replicates). (F) Eluates after IPs of nsp13 wild type (WT) or variants recombinantly expressed in 293T cells, as indicated, were probed with antibodies against Strep II to verify the efficiency of IP and against components of the Fe–S cluster (HSC20, HSPA9, and NFS1) and of the cytoplasmic Fe–S (CIA) assembly machinery (CIAO1, MMS19, and FAM96B). Immunoblots to PCBP1 and BOLA2 are also shown as these proteins were identified in our mass spectrometry analysis and have been reported to deliver iron for cytoplasmic Fe–S cluster assembly (21) (n = 4).
The Intricacies of SARS-CoV-2 Replication
Fe–S cluster incorporation into nsp13 occurs through its interactions with components of the Fe–S biogenesis machinery. (A) Domain organization of nsp13. The ZBD is shown in dark blue, followed by a “stalk” region (light blue), a β-barrel, 1B, in magenta, and two helicase core domains RecA1 (1A) and RecA2 (A2) (19). (B) Structure of the N-terminal zinc-binding domain (ZBD) of nsp13 showing the amino acid residues ligating zinc in the available crystal structure (PDB ID: 6ZSL) (20). A completely conserved LYR-like motif (LYK) in the seven human pathogenic coronaviruses is highlighted in yellow. (C) Proposed model of the chaperone/cochaperone-mediated transfer of nascent Fe–S clusters, assembled on the main scaffold protein, ISCU, through the direct binding of the cochaperone HSC20 to LYR motifs present in recipient apoproteins. ATP hydrolysis by the HSC20-cognate chaperone, HSPA9, is proposed to facilitate cluster transfer to recipient proteins, while concomitantly driving folding of the recipient protein into its final conformation. (D) Multiple sequence alignment of the residues ligating zinc and of the conserved LYR-like motif in nsp13 of the seven human pathogenic coronaviruses. Boxes in the same color identify the amino acid residues in each individual metal-binding site. (E) Representative Coomassie blue staining of pull-down assays performed with purified proteins. Purified nsp13-Strep II (0.25 μg) or the variants wherein either the LYK motif or the cysteine and histidine residues coordinating zinc in the available structures were replaced by alanines or serines, respectively, as indicated, were combined with 0.25 μg of HSC20. Immunoprecipitations (IPs) were performed with Strep II antibody to immunocapture nsp13 proteins. The presence of HSC20 (a.k.a. HSCB) in the eluates after IPs of nsp13 proteins was analyzed by SDS-polyacrylamide gel electrophoresis and Coomassie staining. Aliquots corresponding to 20% of the inputs were run on the gel for comparison (n = 4 biological replicates). (F) Eluates after IPs of nsp13 wild type (WT) or variants recombinantly expressed in 293T cells, as indicated, were probed with antibodies against Strep II to verify the efficiency of IP and against components of the Fe–S cluster (HSC20, HSPA9, and NFS1) and of the cytoplasmic Fe–S (CIA) assembly machinery (CIAO1, MMS19, and FAM96B). Immunoblots to PCBP1 and BOLA2 are also shown as these proteins were identified in our mass spectrometry analysis and have been reported to deliver iron for cytoplasmic Fe–S cluster assembly (21) (n = 4).
The Intricacies of SARS-CoV-2 Replication
The replication of SARS-CoV-2, the virus responsible for COVID-19, is a complex and precisely orchestrated process. Central to this process is the RNA-dependent RNA polymerase (RdRp), an enzyme responsible for copying the v
iral RNA genome. While RdRp has been a primary focus of research, other nonstructural proteins (nsps) within the virus have received limited attention despite their potential significance. Nsp13, a helicase belonging to the helicase superfamily 1B (SF1B), has emerged as a crucial component of the viral replication machinery.
Unlocking the Role of Nsp13: A Paradigm Shift
Initially regarded as a modest helicase in past studies and
COVID-19 News reports, nsp13 has taken center stage with revelations of its intricate involvement in SARS-CoV-2 replication. Researchers uncovered a remarkable synergy between nsp13 and RdRp, enhancing the helicase's activity. This partnership underscores the dynamic interactions between various proteins within the viral replication complex, challenging conventional perceptions of nsp13's function.
Beyond Unwinding: Nsp13's Expanding Portfolio
The significance of nsp13 extends beyond its role in RNA unwinding. Recent studies unveiled its pivotal contribution to the formation of the 5' mRNA cap, a protective structure crucial for safeguarding viral RNA from degradation and facilitating translation within host cells. This newfound role showcases the multifaceted nature of nsp13, positioning it as a central player in the intricate dance of viral replication.
A Glimpse into Nsp13's Molecular Landscape
To unravel the mysteries of nsp13, the study team embarked on a comprehensive journey, harnessing an array of advanced analytical techniques. Techniques including inductively coupled plasma mass spectrometry (ICP-MS), UV-visible absorption, electron paramagnetic resonance (EPR), and Mössbauer spectroscopy provided unprecedented insights into the structural nuances of nsp13. These analyses not only unveiled its architecture but also shed light on its dynamic interactions within the viral replication machinery.
Unraveling the Enigmatic Iron-Sulfur Cluster
Amidst the intricate folds of nsp13's structure, an enigmatic revelation emerged - an iron-sulfur (Fe-S) cluster nestled within its zinc-binding domain (ZBD). This [Fe4S4] cluster, renowned for its pivotal role in diverse biological processes, emerged as a linchpin in nsp13's functionality. The Fe-S cluster bestowed nsp13 with enhanced RNA binding and unwinding abilities, unveiling the choreography orchestrating SARS-CoV-2 replication at the molecular level.
TEMPOL: A Formidable Adversary to Nsp13
In a remarkable turn of events, TEMPOL, a stable nitroxide compound, emerged as a potent adversary to nsp13's Fe-S cluster. TEMPOL's ability to disrupt the Fe-S cluster led to the inhibition of nsp13's helicase activity. This discovery positions TEMPOL as a potential trailblazer for a new class of anti-COVID-19 therapeutics, distinguishing itself from conventional strategies targeting RdRp.
A Glimpse into the Future: An Antiviral Vanguard
As the world confronts the ongoing challenge posed by SARS-CoV-2, the quest for versatile and potent therapeutic agents intensifies. Nsp13's pivotal role in viral replication, coupled with its conservation across diverse coronaviruses, offers the tantalizing prospect of a broad-spectrum antiviral capable of combating not only the current strain but also potential future variants.
TEMPOL: A Ray of Optimism
The impressive efficacy of TEMPOL in disrupting nsp13's Fe-S cluster, combined with its minimal toxicity profile, positions it as a compelling contender in the arsenal of anti-COVID-19 drugs. By integrating TEMPOL with other direct antiviral agents and immune modulators, a comprehensive strategy could be devised to mitigate the severity of the disease in both early and advanced stages of infection.
Conclusion
The current study delved into the intricate facets of nsp13, an essential nonstructural protein found in various coronavirus strains. Employing an array of advanced spectrometric techniques, the researchers embarked on a journey to unveil the protein's structural intricacies and its underlying stoichiometry.
Through these meticulous analyses, a striking revelation emerged - nsp13 wields a pivotal, yet often underestimated, role in the replication of SARS-CoV-2. Its significance becomes particularly evident as it dramatically enhances the RNA unwinding process, triggered by the incorporation of Fe-S from nsp12, the catalytic nucleus of the RdRp complex.
Of noteworthy significance, nsp13 stands as one of the most remarkably conserved segments within the expansive tapestry of the SARS-CoV-2 genome. This groundbreaking study not only unveiled this hidden treasure within the genome but also unveiled a promising warrior against viral intrusion. Enter TEMPOL, a stalwart stable nitroxyl antioxidant, championed for its prowess in inhibiting nsp13. Its potency as a broad-spectrum anti-viral agent emerges as a beacon of hope, further accentuated by its commendable safety profile in human applications. Thus, TEMPOL emerges as an appealing contender for an oral postexposure defense strategy against the relentless SARS-CoV-2.
The study findings were published in the peer reviewed journal: PNAS.
https://www.pnas.org/doi/10.1073/pnas.2303860120
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
