Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 28, 2023 2 years, 3 months, 4 days, 19 hours, 39 minutes ago
COVID-19 News: The COVID-19 pandemic has left an indelible mark on the world, affecting millions of lives and leading to an array of complications that extend beyond the initial infection. One of the most challenging aspects of this pandemic is Long COVID, also known as post-acute sequelae of SARS-CoV-2 infection (PASC). This complex and poorly understood syndrome is characterized by a wide range of persistent symptoms that can last for months after the initial COVID-19 infection. These symptoms encompass fatigue, shortness of breath, cognitive dysfunction, and various other manifestations that significantly impact the daily lives of those afflicted.
Long COVID represents a perplexing and ongoing public health concern. It is recognized by both the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) as a syndrome where common symptoms persist for an extended period after the initial SARS-CoV-2 infection. These symptoms often present in a relapsing and fluctuating manner, making it distinct from other post-viral complications. Long COVID has affected millions of individuals globally, with a staggering estimated number of over 17 million cases in Europe alone.
This
COVID-19 News report delves into a study by a researcher from the Department of Cardiology, Adena Health System, Chillicothe, Ohio-USA which explores the role of Tau proteins in Long COVID.
Pathophysiology
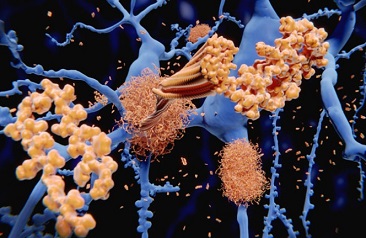
Despite the growing recognition of Long COVID, the underlying pathophysiology remains elusive. Various hypotheses have been proposed to explain the persistence of symptoms, and recent research has shed light on the potential role of tau protein in this enigmatic syndrome. Tau protein is primarily associated with neurodegenerative disorders known as tauopathies, characterized by the accumulation of abnormal tau deposits in the brain. These deposits can affect different regions of the brain and even extend to the peripheral nervous system.
Long COVID presents a wide array of symptoms, exceeding 203 different manifestations. Some of the most common include fatigue, dyspnea (shortness of breath), and cognitive dysfunction. Remarkably, these clinical symptoms bear a striking resemblance to the effects observed in tauopathies, hinting at a potential connection. While the definitive pathophysiology of Long COVID is yet to be established, the hypothesis of aberrant tau protein phosphorylation holds promise for further investigation.
Tau protein plays a crucial role in maintaining the stability of microtubules and facilitating their polymerization in normal physiological conditions. However, in pathological states, tau protein can undergo aberrant phosphorylation, post-translational modifications, and aggregation into insoluble filaments. Studies have demonstrated that this hyper-phosphorylation of tau protein can lead to neuronal cell death, a process that may be pertinent to Long COVID.
Tau Protein & COVID-19
The SARS-CoV-2 virus responsible for COVID-19 is known to affect various tissues throughout the body by binding to the ACE2 receptor. These tissues include cardiac pericytes, blood vessels, respiratory epithelial cells, the kidneys, intestines, and remarkably, the hu
man brain. This multi-organ involvement during the acute phase of the infection is a result of the virus entering the body through ACE2 receptors.
Notably, autopsy studies of COVID-19-affected brains have revealed the presence of diffuse inflammatory markers in over 80% of patients. These inflammatory processes could be a contributing factor to the diverse neurological symptoms associated with the disease. Among the most common neurological symptoms observed are hyposmia (loss of smell) and hypogeusia (loss of taste), both of which have been linked to pathological tau protein in Alzheimer's disease.
A recent study that compared autopsy tissue samples of COVID-19-affected brains to control samples found that oxidative stress and inflammatory pathways may trigger the hyper-phosphorylation of tau protein in COVID-19-affected brains. This study provided critical insights into the potential connection between COVID-19 and tau protein pathology.
Further support for the role of tau protein comes from in vitro studies using human-brain organoids derived from induced human pluripotent stem cells. These studies have shown that SARS-CoV-2 infection can lead to the aberrant phosphorylation of tau protein, which, in turn, initiates a cascade of events resulting in neuronal cell death. These findings highlight the potential link between COVID-19 and tau protein pathology.
Tau Protein & Immunopathology
In COVID-19, immuno-pathological pathways with dysregulated innate immune responses play a pivotal role in disease pathogenesis. Severe cases of COVID-19 are marked by elevated levels of pro-inflammatory cytokines, including IL-1, IL-2, IL-4, IL-6, IL-7, IL-10, IL-13, IL-17, M-CSF, G-CSF, GM-CSF, and IP-10. An essential component of immuno-pathology is the NOD-like receptor protein 3 (NLRP-3) inflammasome, which has been associated with tauopathy and is known to influence tau protein phosphorylation.
Another key player in immunopathology is the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor that regulates inflammatory responses in various conditions, including neurodegenerative disorders like tauopathies. Inhibition of NF-κB has been shown to slow down the propagation of tau pathology in animal models, and there is evidence of SARS-CoV-2 activating NF-κB through various viral proteins.
Tau Protein & Tauopathies
Tau protein is a microtubule-associated protein that plays a critical role in stabilizing microtubules, essential for various neuronal functions. However, in pathological states, tau protein can exhibit aberrant phosphorylation, post-translational modifications, truncation, and aggregation into insoluble filaments. These pathological tau deposits are a hallmark of neurodegenerative diseases known as tauopathies. The spectrum of tauopathies includes disorders such as Alzheimer's disease, progressive supranuclear palsy, corticobasal syndrome, frontotemporal dementias, and chronic traumatic encephalopathy.
The specific clinical symptoms associated with tauopathies depend on the affected regions of the brain, resulting in a diverse clinical profile. Efforts in translational research aim to improve early diagnosis of tauopathies, given their clinical diversity, which presents a diagnostic challenge.
Apart from its role in microtubule stability, tau protein influences various aspects of neuronal growth, axonal sprouting, neurite polarity, neuroplasticity, and morphogenesis. In pathological states, tau protein can exhibit pathological characteristics, including aberrant phosphorylation, post-translational modifications, truncation, and aggregation into oligomers and larger insoluble filaments. Crucially, these pathogenic tau deposits can spread to neighboring cells, promoting the propagation of tau pathology, leading to a range of clinical symptoms depending on the affected organ systems.
Small-Fiber Neuropathy & Long COVID
Small-fiber neuropathy is a term used to describe neuropathies that primarily affect thinly myelinated Aδ-fibers and unmyelinated C-fibers. This condition has diverse etiologies, including immunological diseases, genetic mutations, and secondary causes like diabetes mellitus, vitamin B-12 deficiency, and alcohol abuse. The manifestations of small-fiber neuropathy can range from mixed neuropathy to purely sensory or autonomic neuropathy, depending on the extent of nerve fiber involvement.
Recent studies have illuminated a potential connection between small-fiber neuropathy and Long COVID. Skin biopsies from Long COVID patients who had experienced symptoms for an extended period revealed evidence of small-fiber neuropathy in 62.5% of cases. Studies conducted in the UK identified small-nerve fiber loss in patients with neurological symptoms four weeks after their COVID-19 infection. Additionally, research conducted in France detected dysautonomia in Long COVID patients experiencing fatigue, emphasizing the role of the autonomic nervous system in this syndrome.
The association between small-fiber neuropathy and myalgic encephalitis/chronic fatigue syndrome (ME/CFS) has also been explored. Studies have shown that small-fiber neuropathy can lead to impaired aerobic metabolism, which may contribute to the overwhelming fatigue characteristic of both ME/CFS and Long COVID. De-conditioning, often considered a confounding factor in chronic fatigue patients, can lead to low cardiac output with impaired ventricular compliance and elevated filling pressure. These findings contrast with the observations in patients with low-flow preload failure and small-fiber neuropathy.
Ongoing research is delving deeper into the potential link between small-fiber neuropathy and Long COVID. Small-fiber neuropathy may have a substantial impact on cardiovascular and autonomic function, potentially contributing to symptoms experienced by Long COVID patients, such as orthostatic intolerance and post-exertional malaise.
Potential Treatment Options and Targets for Therapeutic Interventions
Understanding the potential role of tau protein and small-fiber neuropathy in Long COVID's pathophysiology opens up new avenues for therapeutic interventions and treatment targets.
Tau protein undergoes post-translational modifications that can reduce its binding to microtubules, leading to an increased level of cytoplasmic tau proteins. These tau aggregates can assemble, leading to the formation of neurotoxic elements. Therapeutic approaches for tauopathies encompass targeting these post-translational modifications, tau aggregation, and tau clearance through autophagy, often facilitated by lysosomes.
Phosphorylation, a key post-translational modification, can be influenced by protein kinases and phosphatases. Phosphoprotein phosphatase (PP2A), a key phosphatase, accounts for over 70% of tau dephosphorylation. Various clinical trials have tested protein kinases as potential therapeutic agents, with mixed success. Glycogen synthase kinase (G.S.K. 3β), associated with tau phosphorylation at numerous sites, has been a focus of clinical trials but has not yet provided definitive success in reversing the disease process.
Recent trials have explored agents targeting tau aggregation. Compounds like rosmarinic acid, found in Lamiaceae herbs, have shown promise in inhibiting the accumulation of phosphorylated tau protein and improving memory. Resveratrol, a polyphenol found in red wine and grape skin, induces tau dephosphorylation and has demonstrated improvements in cognitive function in animal models. However, its clinical use has been hampered by issues of bioavailability. Analogs with improved bioavailability are under development.
Curcumin, a component of turmeric, has been found to inhibit tau aggregation and reduce tau oligomers. While its bioavailability is a challenge, analogs with better absorption properties have undergone clinical trials. Folic acid has also shown promise in inhibiting tau aggregation and reducing tau phosphorylation. Tau protein degradation involves both the ubiquitin-proteasome system and the autophagy-lysosome system.
Additionally, tau-protein clearance can be enhanced through the use of vaccines or antibodies. Various clinical trials for tauopathies are exploring this approach, with promising results. Synthetic peptides generating antibodies against tau protein, such as AADvac1, are reducing tau pathology in animal models and have shown potential in clinical trials. Humanized monoclonal antibodies, like BIIB092, target specific fragments of tau and are undergoing clinical trials for Alzheimer's disease. Oligonucleotide therapy, which regulates protein expression levels, holds promise for controlling the onset and progression of tauopathies.
Discussion
The post-COVID complications known as Long COVID pose a substantial public health burden. Recent studies have pointed to tau protein deposits in the brains of COVID-19 patients and have shown tau protein hyper-phosphorylation and neuronal cell death in vitro, providing insights into the potential mechanisms underlying this syndrome.
A variety of body stresses, including viral infections, can trigger pathological tau protein. These proteins may then spread to healthier cells through synapses, leading to functional and structural changes in the peripheral and autonomic nervous systems, potentially resulting in peripheral, autonomic, and small-fiber neuropathy.
Long COVID patients predominantly present with symptoms such as fatigue, post-exertional malaise, cognitive dysfunction, peripheral neuropathy, and autonomic dysfunction, which align with the characteristics of tauopathy neuropathy. However, it's important to note that while the potential connection between tau protein and Long COVID is promising, other pathways and factors may also play a role in this emerging syndrome, necessitating further exploration.
Conclusion
To shed more light on the potential role of tauopathy-induced neuropathy among Long COVID patients, a prospective clinical study is proposed. Evaluating cerebrospinal fluid (CSF) for elevated tau protein in human organoids infected with COVID-19 could be the first step toward developing a biomarker for diagnosing Long COVID. Elucidating the underlying mechanisms of aberrant tau protein phosphorylation may help identify new therapeutic targets for Long COVID and other tauopathies.
As we continue to navigate the complexities of the COVID-19 pandemic, unraveling the enigma of Long COVID and understanding its underlying pathophysiology becomes paramount. This knowledge can lead to more effective care and support for those grappling with this perplexing syndrome while providing insights into the broader implications of tau protein in neurodegenerative diseases and beyond.
The study findings were published in the peer reviewed journal: Frontiers in Cellular and Infection Microbiology.
https://www.frontiersin.org/articles/10.3389/fcimb.2023.1280600/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
