COVID-19 News: Study Shows That Exposure To SARS-CoV-2 During Pregnancy Results In Newborns Developing Epigenetic Changes!
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 31, 2023 1 year, 4 months, 4 weeks, 2 days, 15 hours, 10 minutes ago
COVID-19 News: The COVID-19 pandemic, which began in early 2020, has brought unprecedented challenges and stressors to people around the world. From concerns about personal health to the social and economic disruptions, the pandemic has left no aspect of life untouched. This global crisis has not spared pregnant women, who have had to navigate the unique stressors associated with the pandemic. Recent research suggests that the impact of maternal stress during pregnancy can lead to long-term neurodevelopmental and cognitive consequences for the child. In light of these findings, scientists have conducted a groundbreaking study to investigate the epigenetic changes in newborns born to mothers who were exposed to the COVID-19 pandemic during pregnancy.
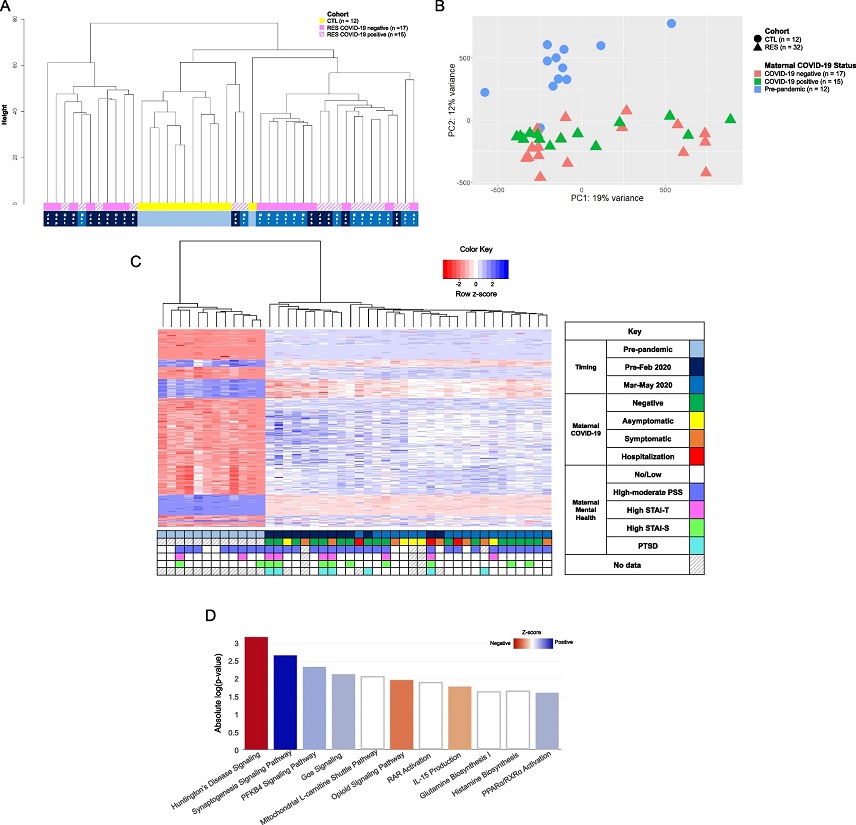 DNA methylation differences associated with newborns exposed to the COVID-19 pandemic in utero. A Dendrogram representation of unsupervised hierarchical clustering of normalized and batch-corrected beta values of pre-pandemic controls (CTL, yellow), RES pandemic COVID-19-negative pregnancies (solid pink), and RES COVID-19-positive pregnancies (hatched pink). Timing of approximate start of gestation is indicated at the bottom: light blue, entirety of pregnancy occurred prior to start of the pandemic (December 2019); dark blue; gestation that started September 2019-February 2020, prior to the onset of the COVID-19 pandemic; medium blue, gestation started between March and May 2020, after the US declared a national disaster. B Principal component analysis (PCA) of global DNA methylation differences of normalized and batch-corrected beta values between newborns exposed to the COVID-19 pandemic during pregnancy (RES, n = 32, triangles) and pre-pandemic healthy controls (CTL, n = 12, circles). Subcategorization by maternal COVID-19 infection status during pregnancy is denoted by color: coral indicates COVID-19 negative pregnancies (n = 17), green indicates COVID-19 positive pregnancies (n = 15), and blue indicates pre-pandemic pregnancies, unexposed to COVID-19 (n = 12). C Heatmap of unsupervised, hierarchical clustering of the differentially methylated probes identified between pre-pandemic CTL and RES pandemic newborns. Z-score scale represents transformed intensity values between differentially methylated probes with red being a negative z-score, white being a z-score of 0, and blue a positive z-score. The grid below the heatmap represents various clinical metrics, including timing of pregnancy (same color code as in 1A), COVID-19 infection status and severity of disease, and prenatal maternal mental health questionnaire data (STAI-S, STAI-T, PSS, PTSD), keyed in the legend to the right of the heatmap. Each column represents a sample, while rows represent specific probes that are differentially methylated between RES and CTL after performing linear regression analysis (FDR p-value ≤ 0.05, log2 fold-change threshold ≥ 1 or ≤ -1). D IPA analysis of annotated differentially methylated probes between CTL and RES cohort. Z-score scale was calculated from differential intensity values and red and blue shades indicate pathways with a negative or positive z-score, respectively. White indicates pathways with a z-score of 0 (genes in the pathway are differentially methylated, some positively, some negatively, resulting in a Z-score = 0).&am
p;nbsp;
DNA methylation differences associated with newborns exposed to the COVID-19 pandemic in utero. A Dendrogram representation of unsupervised hierarchical clustering of normalized and batch-corrected beta values of pre-pandemic controls (CTL, yellow), RES pandemic COVID-19-negative pregnancies (solid pink), and RES COVID-19-positive pregnancies (hatched pink). Timing of approximate start of gestation is indicated at the bottom: light blue, entirety of pregnancy occurred prior to start of the pandemic (December 2019); dark blue; gestation that started September 2019-February 2020, prior to the onset of the COVID-19 pandemic; medium blue, gestation started between March and May 2020, after the US declared a national disaster. B Principal component analysis (PCA) of global DNA methylation differences of normalized and batch-corrected beta values between newborns exposed to the COVID-19 pandemic during pregnancy (RES, n = 32, triangles) and pre-pandemic healthy controls (CTL, n = 12, circles). Subcategorization by maternal COVID-19 infection status during pregnancy is denoted by color: coral indicates COVID-19 negative pregnancies (n = 17), green indicates COVID-19 positive pregnancies (n = 15), and blue indicates pre-pandemic pregnancies, unexposed to COVID-19 (n = 12). C Heatmap of unsupervised, hierarchical clustering of the differentially methylated probes identified between pre-pandemic CTL and RES pandemic newborns. Z-score scale represents transformed intensity values between differentially methylated probes with red being a negative z-score, white being a z-score of 0, and blue a positive z-score. The grid below the heatmap represents various clinical metrics, including timing of pregnancy (same color code as in 1A), COVID-19 infection status and severity of disease, and prenatal maternal mental health questionnaire data (STAI-S, STAI-T, PSS, PTSD), keyed in the legend to the right of the heatmap. Each column represents a sample, while rows represent specific probes that are differentially methylated between RES and CTL after performing linear regression analysis (FDR p-value ≤ 0.05, log2 fold-change threshold ≥ 1 or ≤ -1). D IPA analysis of annotated differentially methylated probes between CTL and RES cohort. Z-score scale was calculated from differential intensity values and red and blue shades indicate pathways with a negative or positive z-score, respectively. White indicates pathways with a z-score of 0 (genes in the pathway are differentially methylated, some positively, some negatively, resulting in a Z-score = 0).&am
p;nbsp;
The study, conducted by researchers from the Center for Genetic Medicine Research at the Children's National Research & Innovation Campus in Washington, USA, along with collaborators from George Washington University, Children’s National Hospital, and the University of California, Irvine, is titled "Genome-wide neonatal epigenetic changes associated with maternal exposure to the COVID-19 pandemic." It aims to shed light on whether maternal exposure to the COVID-19 pandemic can result in unique epigenetic signatures in newborns and, potentially, long-term developmental consequences. The study ad its findings are covered in the
COVID-19 News report.
Background
Maternal stress during pregnancy has long been associated with adverse developmental outcomes in children. This includes conditions such as impaired fetal development and long-term neurodevelopmental and cognitive consequences. Researchers have explored the impact of various stressors, including viral exposure and maternal psychological distress, on the fetus. For instance, past studies have examined the consequences of traumatic events like natural disasters, war, and pandemics on pregnancy and maternal mental health. Notable examples include the Dutch Hunger Winter (1944–45) and the Canadian Ice Storm (1998). Such studies have utilized DNA methylation analysis to uncover how environmental stressors can affect maternal mental health and fetal development.
The present study extends this research by focusing on the specific impact of the COVID-19 pandemic on newborns' epigenomes. The term "epigenome" refers to the set of chemical compounds and proteins that can modify the DNA in our genes and influence their activity. Changes in the epigenome can have profound effects on gene expression and, ultimately, development. Therefore, understanding the impact of the pandemic on the epigenome of newborns is of great importance.
Methods
The research project, known as "Project RESCUE" (Reducing Elevated Stress from COVID-19 Exposure Project), is a prospective observational and longitudinal cohort study. It evaluates how elevated prenatal maternal stress during the COVID-19 pandemic can influence early childhood neurodevelopment. The study compared two cohorts: pre-pandemic controls and pandemic-exposed newborns, both recruited from the Washington DC Metropolitan region.
To analyze the epigenetic changes in newborns, buccal swabs were collected at birth. The researchers employed the Infinium MethylationEPIC arrays and conducted differential DNA methylation analysis using linear regression. Additionally, pathway analysis and gene ontology enrichment were performed on the resulting gene lists.
Results
The study uncovered significant differences in DNA methylation between newborns exposed to the pandemic during gestation and those born before the pandemic. Over 500 annotated sites of differential methylation were identified. Among these, methylation was observed at NR3C1, a glucocorticoid receptor gene. This gene has previously been linked to increased methylation in studies of perinatal stress in newborns, as well as various psychological disorders. These findings align with existing literature, highlighting the influence of prenatal stress on the newborn epigenome.
Furthermore, the researchers found that differential methylation affected critical pathways associated with neurological development and the immune system, emphasizing the potential consequences of maternal exposure to the pandemic on the child's future health and cognitive development. Specific pathways that stood out included IL-15 production, Huntington's disease signaling, synaptogenesis signaling, and the PFKB4 pathway.
The study also delved into the timing of pandemic exposure, distinguishing between infants exposed to the early phase and those exposed to the later stages of the pandemic. Notably, the researchers identified distinct epigenetic signatures in these two groups, suggesting that the timing and duration of stressors during pregnancy may have varying effects on the newborn epigenome.
Discussion
Maternal mental health during pregnancy is a critical factor in fetal development. The stressors brought about by the COVID-19 pandemic, including the fear of infection, economic instability, and social isolation, have had a significant impact on the mental health of pregnant women. As a result, Project RESCUE aimed to investigate the epigenetic changes in newborns born to mothers who experienced these unique stressors.
The researchers found that maternal exposure to the pandemic resulted in widespread differences in DNA methylation in newborns. Importantly, these differences were not linked to maternal COVID-19 infection status, implying that the changes were a direct consequence of the pandemic's environment.
One of the key genes affected by this differential methylation was NR3C1, associated with stress and various psychological disorders. This finding underscores the critical role of maternal stress during pregnancy in shaping the epigenome of the child.
Pathway analysis also revealed the involvement of several critical pathways related to neurological development and immune system response. The upregulation of IL-15 production, which was observed in the pandemic-exposed newborns, has been linked to increased inflammation and adverse outcomes in pregnancy. However, it is noteworthy that this pathway was more methylated in newborns exposed to the pandemic, which could be a protective mechanism against negative developmental outcomes.
The study also highlighted pathways associated with neurodegenerative diseases, such as Huntington's disease signaling and synaptogenesis signaling. These pathways are critical for healthy neurodevelopment, and their dysregulation has been associated with impaired neurodevelopment in newborns. The findings suggest that the stressors experienced during pregnancy, including the COVID-19 pandemic, may impact these pathways, leading to long-term consequences.
The PFKB4 pathway, which regulates glycolysis and ectodermal patterning during embryonic development, was also affected. This pathway's impact on developmental outcomes remains to be explored.
The study further revealed distinct epigenetic signatures in newborns exposed to the early and late phases of the pandemic. This temporal aspect highlights the importance of considering when stressors occur during pregnancy and their duration, as it can influence postnatal outcomes for children.
Conclusion
In conclusion, the research conducted by the Center for Genetic Medicine Research and its collaborators sheds light on the profound impact of maternal exposure to the COVID-19 pandemic on the epigenome of newborns. The study revealed widespread differences in DNA methylation, with over 500 sites showing significant changes, including the critical gene NR3C1 and pathways associated with neurological development and the immune system.
Notably, the timing of pandemic exposure during pregnancy played a significant role in the epigenetic changes observed in newborns. Those exposed to the early and late phases of the pandemic exhibited distinct signatures, emphasizing the importance of understanding when stressors occur during gestation.
While the study had its limitations, including sample size and potential confounding factors, its findings provide crucial insights into the potential long-term consequences of maternal stress during the COVID-19 pandemic. Understanding these epigenetic changes is a critical step in developing strategies to support the health and well-being of both mothers and their children during and after challenging times like the COVID-19 pandemic.
This research underscores the importance of considering the impact of environmental stressors on fetal development and the potential for lasting effects on a child's health and cognitive development. It also highlights the need for continued research in this area to further our understanding of the complex relationship between maternal stress, the epigenome, and long-term health outcomes for children.
The study findings were published in the peer reviewed journal: BMC Medical Genomics.
https://bmcmedgenomics.biomedcentral.com/articles/10.1186/s12920-023-01707-4
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-news-covid-19-exposure-during-pregnancy-leads-to-epigenetic-changes-in-newborns,-paving-the-way-for-health-issues-later-in-life
https://www.thailandmedical.news/news/breaking-news-covid-19-infections-during-pregnancy-alters-expression-of-important-pregnancy-genes-that-can-give-rise-to-complications
https://www.thailandmedical.news/news/study-shows-disruptions-in-lipid-metabolism-in-covid-19-infections-during-pregnancy-will-affect-newborn-health
https://www.thailandmedical.news/news/breaking-news-offspring-of-pregnant-women-with-covid-19-will-likely-have-altered-brain-developments-especially-males
https://www.thailandmedical.news/news/breaking-news-covid-19-infection-in-pregnant-women-alters-microbiota-composition-passed-onto-infants,-impacting-their-long-term-health
