COVID-19 News: Study Shows That Literally All The Different Structural Proteins Of SARS-CoV-2 Play A Role In Causing Lung Injury
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 05, 2024 1 year, 2 months, 1 week, 3 days, 18 hours, 22 minutes ago
COVID-19 News: In the ongoing battle against the COVID-19 pandemic, researchers are uncovering intricate details about the SARS-CoV-2 virus that go beyond its surface. A groundbreaking study covered in this
COVID-19 News report conducted at Shaoxing Second Hospital in Zhejiang, China, and The Children’s Hospital of Zhejiang University School of Medicine, in collaboration with the National Clinical Research Center for Child Health, has revealed a significant revelation: every structural protein of SARS-CoV-2 plays a pivotal role in causing lung injury. This finding has far-reaching implications, prompting a deeper examination of the structural features, mechanisms of inflammatory response, and the ability of these proteins to independently induce lung injury.
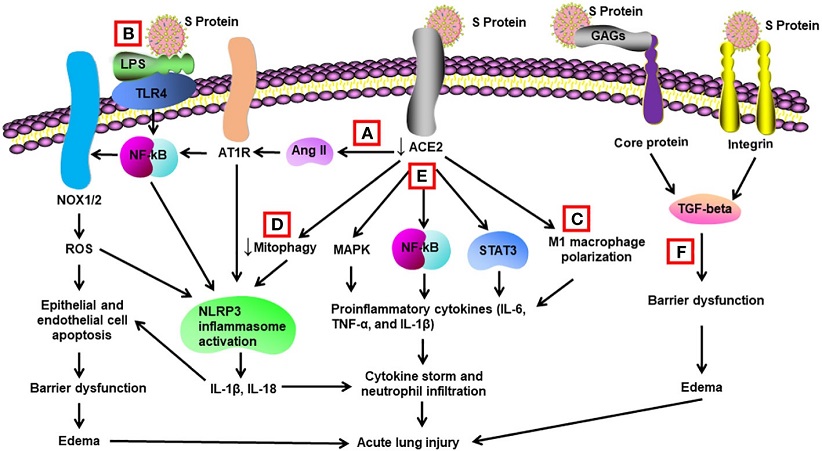 Schematic diagram of mechanisms for S protein-induced lung injury.
Schematic diagram of mechanisms for S protein-induced lung injury.
(A) S protein binds with ACE2, leading to ACE2 shedding and downregulation. Reduced ACE2 promotes the activation of Ang II/angiotensin II type 1 receptor (AT1R)/NF-κB/NOX1/2 pathway, leading to generation of reactive oxygen species (ROS). ROS enhances apoptosis of alveolar epithelial cells and endothelial cells, resulting in barrier dysfunction and edema. Activated AT1R also activates NLR family pyrin domain containing 3 (NLRP3) inflammasome in alveolar macrophages and triggers cell apoptosis and cytokine storm. (B) S protein activates Toll-like receptors such as TLR4 through binding of lipopolysaccharide (LPS) and enhances NF-κB activity, resulting in activation of NLRP3 inflammasome and cytokine storm. (C) S protein promotes M1 polarization of alveolar macrophages through binding to ACE2, which enhances the proinflammatory response. (D) S protein reduces mitophagy through binding to ACE2, resulting in activation of NLRP3 inflammasome and cell apoptosis. (E) S protein binds to ACE2 and triggers activation of STAT3, MAP kinases (MAPK), and NF-κB via other unidentified mechanisms, leading to cytokine storm and neutrophil infiltration. (F) S protein triggers vascular leak and edema independent of ACE2 binding. S protein binds with glycosaminoglycans and integrins, which leads to the activation of TGF-β signaling pathway and barrier dysfunction.
Structural Insights into SARS-CoV-2 Proteins
SARS-CoV-2, with its single-stranded positive RNA genome of approximately 30 kb, encodes four key structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). These proteins are vital for the virus's infectious cycle, including host cell entry, genome replication, assembly, and release of viral particles.
Spike Protein (S): The S protein, responsible for mediating viral attachment to target cells through angiotensin-converting enzyme 2 (ACE2) binding, has two subunits (S1 and S2). Mutations in the S protein, such as the Alpha, Beta, Gamma, Delta, and Omicron variants, have been linked to increased infectivity and immune evasion.
Envelope Protein (E): As the smallest structural protein, E is indispensable in viral assembly, release, and pathogenesis. It forms cation-selective channels, viroporins, contributing to inflammatory responses and disruption of tight junctions in the lungs.
Membrane Protein (M): Essential for viral assembly, M protein binds weakly with N protein and plays a crucial role in coordinating the assembly of SARS-CoV-2 virions. Its interaction with E protein enhances virion release.
Nucleocapsid Protein (N): N protein packages the viral genomic RNA, promotes RNA transcription, and plays a role in immune responses. N protein has been implicated in inducing acute lung injury through various pathways, including binding with the receptor for advanced glycation end-products (RAGE).
Structural Protein-Induced Inflammatory Responses In Vitro
Understanding the mechanisms underlying the inflammatory responses triggered by SARS-CoV-2 structural proteins is crucial. Each protein contributes uniquely to this process:
Spike Protein (S): S protein induces inflammatory responses by promoting ACE2 shedding, downregulating ACE2 expression, and activating pathways such as NF-κB and NLRP3 inflammasome. Toll-like receptors (TLRs) and other receptors, such as TLR2, TLR4, and TLR7, have been implicated in S protein signaling, leading to the release of proinflammatory cytokines.
Envelope Protein (E): E protein, through viroporin activity, activates NLRP3 inflammasome, triggers inflammation, and disrupts tight junctions. Its binding to integrins and activation of TLR2 contribute to endothelial hyperpermeability and inflammation.
Membrane Protein (M): M protein modulates interferon production, inhibits IFN-β promoter activation, and induces cell apoptosis. It has been linked to endothelial hyperpermeability and apoptosis of lung cells.
Nucleocapsid Protein (N): N protein induces inflammatory responses by binding with various cellular proteins, promoting liquid-liquid phase separation, and triggering the release of proinflammatory cytokines. It also suppresses the innate immune response through interactions with TRIM25 and G3BP1.
Structural Protein-Evoked Lung Injury in VIvo
The most striking revelation from this study is that SARS-CoV-2 structural proteins, independently of intact virus presence, can induce lung injury in vivo.
Here's a breakdown of the impact of each structural protein:
Spike Protein (S): Administration of recombinant S protein in transgenic mice overexpressing human ACE2 led to acute lung injury, elevated inflammatory cytokines, and histological signs of lung damage. The S1 subunit of S protein exacerbated lung injury in mice on an alcohol diet, activating NF-κB, STAT3, and NLRP3 pathways.
Envelope Protein (E): Intratracheal administration of recombinant E protein induced lung inflammation, inflammatory cell accumulation, and cell death in wild-type mice. Blockage of TLR2, a receptor for E protein, reduced virus-induced mortality and inflammation.
Membrane Protein (M): M protein, through interactions with BCL-2 ovarian killer (BOK), promoted mitochondrial apoptosis and lung cell apoptosis in vivo. Lentiviral expression of M protein elevated pulmonary permeability and induced apoptosis.
Nucleocapsid Protein (N): N protein administration in mice resulted in acute lung injury, increased protein permeability, proinflammatory cytokine release, and infiltration of neutrophils. N protein-induced lung injury was mediated through RAGE-ERK1/2-NF-κB pathway activation.
Conclusions
This comprehensive study sheds light on the intricate interplay between SARS-CoV-2 structural proteins and the induction of lung injury. The findings underscore the importance of these proteins beyond viral entry, emphasizing their ability to elicit inflammatory responses and cause damage independently. The implications for COVID-19 treatment strategies are profound, as recognizing the mechanisms behind structural protein-induced lung injury paves the way for targeted therapeutic interventions. Antibody cocktails targeting these structural proteins could emerge as a promising avenue for treating severe cases of COVID-19. As the scientific community delves deeper into the molecular intricacies of SARS-CoV-2, this study marks a significant step forward in our understanding of the virus's pathogenesis and potential treatment modalities.
The study findings were published in the peer reviewed journal: Frontiers in Immunology.
https://www.frontiersin.org/articles/10.3389/fimmu.2024.1332440/full
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
