COVID-19 News: Swiss Study Uncovers How SARS-CoV-2 Manipulates Cell Damage Response For Enhanced Viral Fusion
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 15, 2023 1 year, 4 months, 4 weeks, 2 hours, 50 minutes ago
COVID-19 News: In the relentless pursuit of understanding the intricate interplay between viruses and their host cells, a groundbreaking study conducted at the Global Health Institute, School of Life Sciences, EPFL, Lausanne, Switzerland, has shed light on a captivating aspect of SARS-CoV-2 infection. The study covered in this
COVID-19 News report, reveals that the virus, responsible for the global COVID-19 pandemic, manipulates a cell damage response pathway to optimize the activity of its fusion glycoprotein, Spike. This discovery adds a new layer of complexity to our comprehension of viral-host interactions, offering potential insights into therapeutic strategies and further emphasizing the need for a holistic understanding of viral pathogenesis.
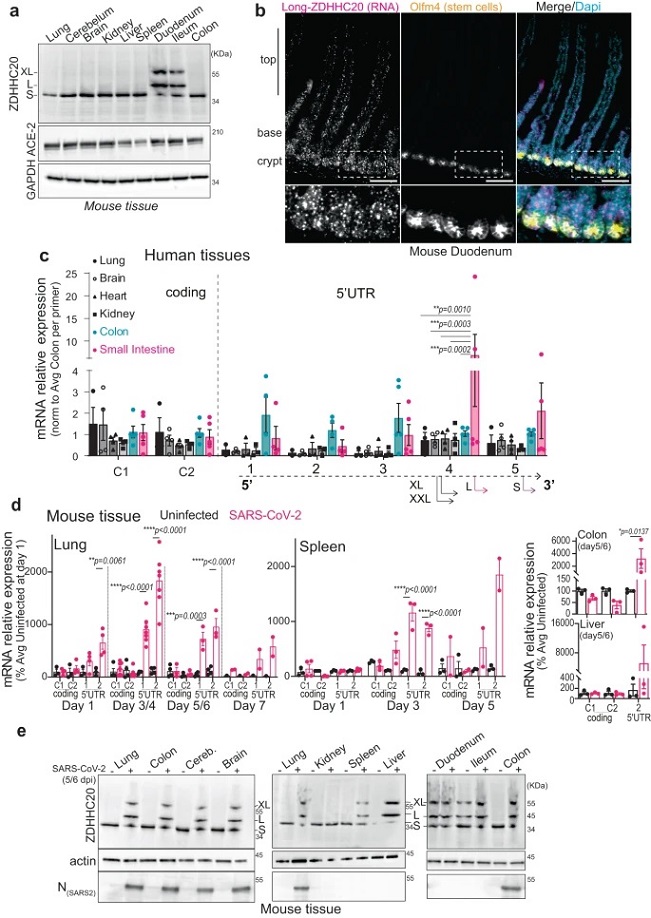 SARS-CoV-2 infection triggers expression of ZDHHC20Long in mice.
SARS-CoV-2 infection triggers expression of ZDHHC20Long in mice.
a WB of ZDHHC20, ACE-2, and GAPDH loading control in mouse tissue extracts. Short (44 KDa, S), Long (50 KDa, L), and XL (53 KDa) ZDHHC20 forms are indicated. b Longitudinal sections of mouse duodenum are labeled with an RNAscope probes for 5’ UTR zdhhc20 RNA, DAPI, and antibodies against Olfm4 (marking intestinal stem cells). Scale bars 100 µm. c mRNA quantification in human tissues, using primers probing for different locations in zdhhc20 transcripts (coding region: C1, C2; 5’ UTR: 1–5 cover increasing lengths of 5’ ends see also Supplementary Fig. 1d). Results are mean ± SEM and each dot represents one independent pool of human-derived tissue-specific mRNA. Lung, n = 3, Brain and Heart, n = 4, and Colon and Small Intestine, n = 5. P values comparing primer-specific expression between tissues were obtained by two-way ANOVA with Tukey’s multiple comparison. d mRNA quantification in different murine tissues uninfected or infected intranasally with 103-104 (plaque-forming units—PFU) of SARS-CoV-2. Tissues were harvested at indicated times post infection and the extracted mRNA analyzed using primers for different locations in zdhhc20 transcripts (coding region (C1, C2) or the 5’ UTR [1–2] see also Supplementary Fig. 2c. e WB analysis of tissue extracts from control uninfected mice or mice infected as in (d). Tissues were harvested at 5–6 days post infection. Actin was used as loading control.). Results are mean ± SEM and each dot represent one of 4 independent mice. P values were obtained by two-way ANOVA with Sidak’s multiple comparison. For all *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, and source data are provided as a Source Data file or Supplementary information.
The Fusogenic Interplay of SARS-CoV-2
SARS-CoV-2 gains entry into host cells through the intricate interplay between its trimeric envelope glycoprotein, Spike, and cellular receptors like ACE-2. This fusion process, essential for infection
, relies on the post-translational modification of Spike through S-acylation, a process where a fatty acid is attached to cysteine residues in its cytoplasmic tail. The primary orchestrator of this modification is the host enzyme ZDHHC20, with contributions from ZDHHC9.
Previous observations hinted at the augmented S-acylation of Spike during SARS-CoV-2 infection compared to Spike transfection alone. The Swiss study delves into the molecular nuances of this phenomenon, uncovering a pivotal mechanism. SARS-CoV-2 infection induces a change in the transcriptional start site of the zdhhc20 gene, leading to the production of an N-terminally extended enzyme, aptly named ZDHHC20Long. This extended version boasts a 67-amino-acid extension, exhibiting a staggering 40 times higher Spike acylating activity than its conventional counterpart.
Remarkably, the study finds that the transcriptional shift in zdhhc20 is not exclusive to SARS-CoV-2 infection. Other cellular challenges, such as chemically induced colitis and exposure to pore-forming toxins, trigger a similar transcriptional change. This discovery suggests that SARS-CoV-2 cunningly exploits an existing cell damage response pathway to optimize its fusion glycoprotein, emphasizing the virus's ability to hijack and repurpose cellular mechanisms for its benefit.
Results Unveiled - SARS-CoV-2's Influence on ZDHHC20
The comparative analysis of Spike S-acylation during infection versus transfection unravels intriguing insights. While Spike transfection yields detectable S-acylation, it pales in comparison to the pronounced S-acylation observed during SARS-CoV-2 infection. This discrepancy led the researchers to investigate the underlying molecular alterations driving this phenomenon.
The study reveals that SARS-CoV-2 triggers a shift in the transcriptional start site of zdhhc20, resulting in the production of ZDHHC20Long. This extended enzyme is not limited to in vitro models but is also expressed in an in vivo infection model involving human ACE-2 transgenic mice. The expression of ZDHHC20Long in various tissues of infected mice, excluding the brain and cerebellum, showcases the systemic impact of SARS-CoV-2 on the host's enzymatic machinery.
Beyond Viral Infection - ZDHHC20Long in Colitis and Toxin Exposure
To further probe the versatility of this transcriptional shift, the study explores its occurrence in response to chemically induced colitis and exposure to pore-forming toxins. Intriguingly, ZDHHC20Long expression is detected in mouse colon tissue undergoing recovery after chemically induced colitis, highlighting the potential role of this extended enzyme in cellular repair processes.
Pore-forming toxins, a diverse class of bacterial proteins, also induce the transcriptional shift, suggesting a broader role for ZDHHC20Long in a general damage repair pathway. This observation prompts the researchers to hypothesize that ZDHHC20Long expression may be part of a universal response to danger or damage, requiring further exploration.
The Extended Repertoire of ZDHHC20Long - ER Localization and Extended Half-Life
Intriguingly, ZDHHC20Long exhibits distinctive characteristics compared to its conventional counterpart. The N-terminal extension leads to an impressive fivefold increase in protein abundance due to a prolonged half-life. Furthermore, ZDHHC20Long displays exclusive localization to the Endoplasmic Reticulum (ER), in contrast to the conventional ZDHHC20, which accumulates in the Golgi.
Detailed analyses reveal that a specific retention motif, PERW, within the N-terminal extension is responsible for ER localization. This novel insight raises questions about the functional implications of this altered subcellular distribution and how it contributes to the enzyme's enhanced activity.
ZDHHC20Long's Impact on Spike Modification
The study meticulously investigates the consequences of ZDHHC20Long's expression on Spike modification. In cells expressing ZDHHC20Long, 3H-palmitate incorporation into Spike is significantly faster and reaches levels approximately 37 times higher than in cells expressing the conventional ZDHHC20Short. This enhanced acylation has profound implications for Spike's fusogenic capacity, as acylation significantly influences viral infectivity.
Furthermore, ZDHHC20Long's impact on Spike goes beyond mere acylation. The extended enzyme proves to be a more potent guardian against deacylation, reducing Spike deacylation by approximately half compared to the conventional ZDHHC20Short. This finding suggests a potential mechanism by which the virus ensures the stability of its fusion glycoprotein.
Enhanced Viral Fusion and Infectivity - ZDHHC20Long in Action
The study extends its investigation to the consequences of ZDHHC20Long's expression on SARS-CoV-2 infectivity. Viral particles produced in cells expressing ZDHHC20Long exhibit a twofold increase in viral RNA levels, indicative of enhanced infectivity. This observation holds significant implications for understanding the dynamics of viral replication and may open avenues for therapeutic interventions targeting ZDHHC20Long.
Future Directions and Concluding Thoughts
As the study unfolds the intricate interplay between SARS-CoV-2 and host cells, numerous questions arise, providing exciting directions for future research. Does acylation of Spike enhance its incorporation into nascent virions? What is the role of ZDHHC20Long in the repair and recovery phase of cells, and how does it contribute to the host's defense mechanisms during viral infection?
In conclusion, this Swiss study not only expands our understanding of the molecular intricacies of SARS-CoV-2 infection but also highlights the virus's ability to orchestrate host cellular machinery for its benefit. As the global scientific community continues to unravel the complexities of viral pathogenesis, this study serves as a beacon, guiding us toward a more comprehensive comprehension of the intricate dance between viruses and their hosts.
The study findings were published in the peer reviewed journal: Nature Communications.
https://www.nature.com/articles/s41467-023-43027-2
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
