COVID-19 News: Taiwanese Study Unravels The Role Of Platelets-Derived MicroRNAs In Hyperactive NETs Formation During COVID-19
Nikhil Prasad Fact checked by:Thailand Medical News Team Nov 02, 2023 1 year, 5 months, 2 weeks, 4 days, 16 hours, 3 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has brought about an unprecedented global health crisis. The clinical spectrum of COVID-19 is diverse, ranging from asymptomatic cases to severe and life-threatening forms of the disease. Among the severe cases, there is a dysregulated hyperactivation of the immune system, characterized by an abnormal cytokine immune response, leading to severe complications. One of the crucial factors in the pathogenesis of severe COVID-19 is the formation of hyperactive neutrophil extracellular traps (NETs).
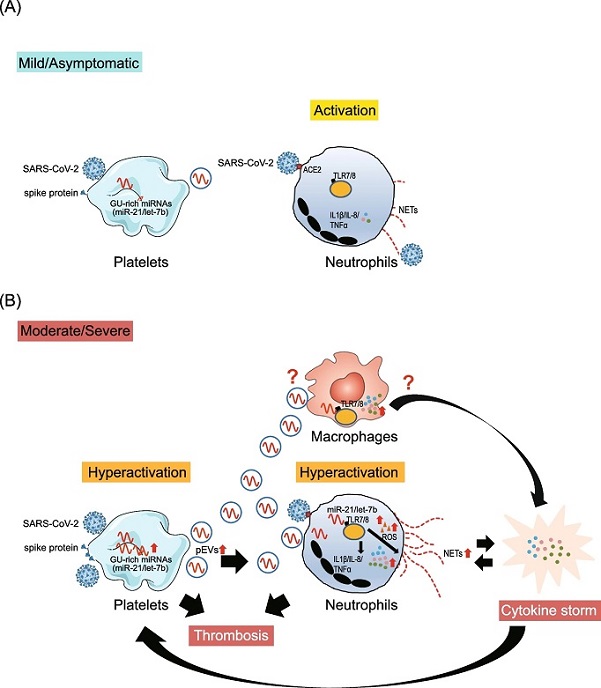 Proposed model for the biological role of platelet–derived miR-21, TLR7/8 and NETs formation in (A) mild COVID-19 and (B) severe COVID-19, based on the results of this study. A SARS-CoV-2 activates neutrophils and induces NETs formation during infection. B In severe COVID-19 cases with platelets hyperactivation, endogenous GU-enriched miRNAs (e.g., miR-21 and let-7b) were upregulated and EVs were increased. The pEVs-carried miR-21/let-7b interacts with TLR7/8 to (1) promote ROS production, thus enhancing SARS-CoV-2 primed NETs formation and (2) activate NF-κB, thus upregulating proinflammatory cytokines/chemokines (IL-1β/TNF-α and IL-8). Increased proinflammatory cytokines/chemokines (e.g., TNF-α) could enhance platelet activation via positive feedback
Proposed model for the biological role of platelet–derived miR-21, TLR7/8 and NETs formation in (A) mild COVID-19 and (B) severe COVID-19, based on the results of this study. A SARS-CoV-2 activates neutrophils and induces NETs formation during infection. B In severe COVID-19 cases with platelets hyperactivation, endogenous GU-enriched miRNAs (e.g., miR-21 and let-7b) were upregulated and EVs were increased. The pEVs-carried miR-21/let-7b interacts with TLR7/8 to (1) promote ROS production, thus enhancing SARS-CoV-2 primed NETs formation and (2) activate NF-κB, thus upregulating proinflammatory cytokines/chemokines (IL-1β/TNF-α and IL-8). Increased proinflammatory cytokines/chemokines (e.g., TNF-α) could enhance platelet activation via positive feedback
Neutrophils, a type of white blood cell, are vital components of the immune system and play a central role in combating infections. They release NETs, which are extracellular webs composed of chromatin, microbicidal proteins, and oxidant enzymes, to capture and eliminate pathogens. However, in the context of COVID-19, excessive NETs formation has been associated with harmful coagulopathy and immunothrombosis, contributing to severe complications such as acute respiratory distress syndrome (ARDS).
In
COVID-19 News report, we delve into a recent study conducted by researchers at Taichung Veterans General Hospital and National Chung Hsing University in Taiwan, which sheds light on the mechanism of SARS-CoV-2-induced NETs formation, particularly the role of platelet-derived extracellular vesicles (EVs) and microRNAs (miRNAs).
The Role of Extracellular Vesicles (EVs) in NETs Formation
Extracellular vesicles (EVs) are small membrane-bound vesicles released by cells that carry various cellular components, including proteins and nucleic acids, to facilitate intercellular communication. Recent research has highlighted the association between EVs derived from severe COVID-19 patients and their ability to promote neutrophil adhesion and induce NETs production.
Platelets are known to be a significant source of circulating EVs and have been implicated in thrombosis. They also play a crucial role in interacting with viruses and are responsible for releasing inflammatory mediators. Studies have shown increased levels of platelet-derived EVs (pEVs) in the blood of COVID-19 patients. Therefore, the researchers hypothesized that SARS-CoV-2 induces platelets to produce specific components, such as microRNAs, which may regulate NETs formation throu
gh pEV transmission.
The study aimed to explore the role of pEVs in hyperactive NETs formation in severe COVID-19 cases, which may provide insights into potential therapeutic strategies.
Platelet Activation and NETs Formation
The study revealed that SARS-CoV-2's spike protein could directly activate platelets, leading to an increase in the release of pEVs in a dose-dependent manner. Moreover, the presence of spike protein-primed pEVs significantly enhanced NETs formation. This finding suggests that pEVs play a crucial role in the dysregulated NETs formation observed in severe COVID-19.
Furthermore, it was found that the spike protein-induced NETs formation was largely dependent on Toll-like receptor 8 (TLR8) and NADPH oxidase, an enzyme complex involved in the generation of reactive oxygen species (ROS). The researchers observed that TLR8 activation contributed more to ROS production and NETs formation than TLR7 in neutrophils.
MicroRNAs and NETs Formation
MicroRNAs (miRNAs) are noncoding RNAs that regulate gene expression by targeting mRNA molecules for degradation or translational repression. These molecules have been shown to exert various biological functions by being carried within EVs to modulate the host immune response during viral infections.
In this study, miR-21 and let-7b, two miRNAs that are abundant in platelets, were found to be upregulated in EVs derived from COVID-19 patients. Additionally, the levels of these miRNAs carried by EVs were associated with the severity of the disease, suggesting that they could serve as potential predisposing factors for severe COVID-19.
The study further revealed that miR-21 and let-7b are GU-enriched miRNAs, and previous research had indicated that GU-rich elements are crucial for innate immune activation by single-stranded RNAs. MiR-21 and let-7b were known to activate TLR8 and TLR7, respectively, but their specific roles in NETs formation remained elusive.
The researchers found that pEVs carrying miR-21/let-7b induced NETs formation in neutrophils through TLR7/8 activation. Notably, TLR8 played a more significant role in this process. They demonstrated that miR-21/let-7b interacted with TLR8 to promote ROS production in neutrophils, ultimately enhancing NETs formation. These findings suggest that miR-21 inhibitors could be potential therapeutic candidates for SARS-CoV-2 infection.
Proinflammatory Cytokines and NETs Formation
The dysregulated production of proinflammatory cytokines is a hallmark of severe COVID-19. Previous studies have shown that proinflammatory cytokines such as IL-1β, IL-8, and TNFα can enhance NETs formation by activating NADPH oxidase and myeloperoxidase (MPO). Elevated levels of IL-8 have been detected in COVID-19 patients, particularly in those with severe disease.
The study demonstrated that COVID-19 patient-derived EVs and viral spike protein-primed pEVs carrying miR-21/let-7b induced the upregulation of proinflammatory cytokines (IL-1β, IL-8, and TNFα) in neutrophils. This effect was mediated through the interaction of miR-21/let-7b with TLR7/8, leading to the activation of NF-κB, a key transcription factor involved in immune responses.
The researchers hypothesized that these pEVs-primed autocrine proinflammatory cytokines may play crucial roles in enhancing NETs formation. These findings open the door to potential therapeutic strategies that target the regulation of proinflammatory cytokines and NETs formation in severe COVID-19 cases.
Conclusion
In conclusion, this study offers valuable insights into the association between platelet-derived miRNAs, NETs formation, and SARS-CoV-2 infection. The discovery of the miR-21-TLR8 axis as a key driver of NETs formation provides a potential therapeutic target for severe COVID-19.
Host-directed therapies have emerged as a promising approach for the treatment of COVID-19. EVs, which play a pivotal role in intercellular communication and immune regulation, represent novel targets for therapeutic interventions. The use of TLR8 antagonists, NADPH oxidase inhibitors, or miR-21 inhibitors may hold promise in mitigating the harmful effects of excessive NETs formation and proinflammatory responses in severe COVID-19 cases.
While this study provides essential groundwork for understanding the molecular mechanisms underlying NETs formation in COVID-19, further research, including in vivo experiments, is needed to validate and expand upon these findings. Nevertheless, the potential implications for therapeutic strategies are exciting and offer hope for improved outcomes in severe COVID-19 cases.
The study findings were published in the peer reviewed journal: Cell Communication and Signaling.
https://biosignaling.biomedcentral.com/articles/10.1186/s12964-023-01345-4
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
