COVID-19 News: University Of Texas Study Shows That SARS-CoV-2 Uses Nonstructural Protein 16 To Evade Restriction By IFIT1 and IFIT3.
COVID-19 News - SARS-CoV-2 - Nsp16- IFIT1- IFIT3 Feb 13, 2023 2 years, 10 months, 1 week, 1 day, 10 hours, 51 minutes ago
COVID-19 News: A new study by researchers from University of Texas Medical Branch, Galveston, Texas-USA, Johns Hopkins University, Maryland-USA and University of California, San Diego, California-USA has found that the SARS-CoV-2 coronavirus uses its nonstructural protein 16 (Nsp16) to evade immunity restrictions by the antiviral proteins: Interferon-induced proteins with tetratricopeptide repeats 1 and 3 ie IFIT1 and IFIT3.
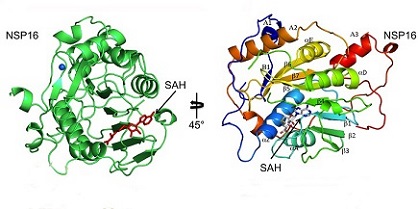
As detailed by previous
COVID-19 News coverages, IFITs are among the most abundantly expressed proteins of the group of
interferon-stimulated genes or ISGs.They represent innate immune effector molecules that confer antiviral defense downstream of type I IFN through disruption of the host translation initiation machinery.
Comprehending the molecular basis of innate immune evasion by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an important consideration for designing the next wave of therapeutics.
The study team investigate the role of the nonstructural protein 16 (NSP16) of SARS-CoV-2 in infection and pathogenesis.
NSP16 is a ribonucleoside 2′-O-methyltransferase (MTase) that catalyzes the transfer of a methyl group to mRNA as part of the capping process.
From observations of other coronaviruses, the study team hypothesized that NSP16 2′-O-MTase function protects SARS-CoV-2 from cap-sensing host restriction.
Hence, the study team engineered SARS-CoV-2 with a mutation that disrupts a conserved residue in the active site of NSP16.
The team subsequently showed that this mutant is attenuated both in vitro and in vivo, using a hamster model of SARS-CoV-2 infection.
Mechanistically, the study findings confirm that the NSP16 mutant is more sensitive than wild-type SARS-CoV-2 to type I interferon (IFN-I) in vitro.
Also, silencing IFIT1 or IFIT3, IFN-stimulated genes that sense a lack of 2′-O-methylation, partially restores fitness to the NSP16 mutant.
The study team also demonstrated that sinefungin, an MTase inhibitor that binds the catalytic site of NSP16, sensitizes wild-type SARS-CoV-2 to IFN-I treatment and attenuates viral replication.
The study findings highlight the importance of SARS-CoV-2 NSP16 in evading host innate immunity and suggest a target for future antiviral therapies.
The study findings were published in the peer reviewed Journal of Virology.
https://journals.asm.org/doi/10.1128/jvi.01532-22
It was highlighted that similar to other coronaviruses, disruption of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) NSP16 function attenuates viral replication in a type I interferon-dependent manner. In vivo, the study findings showed reduced disease and viral replica
tion at late times in the hamster lung, but an earlier titer deficit for the NSP16 mutant (dNSP16) in the upper airway.
The study findings also confirmed a role for IFIT1 but also demonstrate the necessity of IFIT3 in mediating dNSP16 attenuation.
Importantly, the study findings showed that targeting NSP16 activity with a 2′-O-methyltransferase inhibitor in combination with type I interferon offers a novel avenue for antiviral development.
After having established a critical role for NSP16 in evading IFIT activity, the study team evaluated the feasibility of targeting 2′-O-methylation of coronaviruses therapeutically.
Utilizing sinefungin, a pan-inhibitor of SAM-dependent MTases, the researchers surprisingly observed a dose-dependent reduction in replication of WT SARS-CoV-2 in Vero E6.
Since the study team had not observed a replication defect with dNSP16 in Vero E6, it is possible that sinefungin acts beyond NSP16 alone.
Importantly, a pan-MTase inhibitor, sinefungin may also impact the guanine-N7 MTase function of NSP14, which is also vital for viral RNA capping.
Alternatively, sinefungin may also act on a host cell MTase(s), which have been recently implicated in SARS-CoV-2 RNA capping.
https://pubmed.ncbi.nlm.nih.gov/36285486/
While further studies are necessary to differentiate this effect, the study findings indicate that targeting SAM-dependent MTases impairs successful SARS-CoV-2 replication.
Significantly, combined treatment with sinefungin and IFN-I had an additive effect, resulting in increased attenuation likely due to both a loss of viral 2′-O-methylation and increased expression of IFIT1/IFIT3.
Such findings correspond to a similar study with SAM cycle inhibitors that saw the same effect.
https://pubmed.ncbi.nlm.nih.gov/35833542/
Although IFN-I treatments (both IFNα and IFNβ) have shown significant impacts in randomized clinical trials, the study findings suggest that combining such treatments with an NSP16-targeting drug could enhance their impact by reducing toxicity by lowering the therapeutic dose.
https://pubmed.ncbi.nlm.nih.gov/32275914/
Importantly, targeting NSP16 MTase function involves a mechanism distinct from other coronavirus therapies targeting the viral polymerase or the main protease to arrest virus replication.
https://pubmed.ncbi.nlm.nih.gov/32253226/
https://pubmed.ncbi.nlm.nih.gov/35835291/
It was found that in each case, a viral enzymatic process is disrupted; yet for NSP16 targeting, the effector response is provided by the host immune response via IFIT proteins.
Importantly, while attenuation of dNSP16 is delayed in the hamster lung, early attenuation in the nasal washes suggests more rapid or robust expression of IFIT proteins in the upper airway. This could, in turn, increase the efficacy of drugs targeting coronavirus 2′-O-MTase activity in the upper airway, a possible strategy to decrease transmission.
With augmented upper airway replication, a feature of SARS-CoV-2 variants of concern, NSP16-targeting drugs may provide an effective countermeasure for the current and future coronaviruses.
https://pubmed.ncbi.nlm.nih.gov/34818667/
The study findings confirm the importance of NSP16 to SARS-CoV-2 infection and pathogenesis. A mutation that disrupts the NSP16 2′-O-MTase catalytic site attenuates disease in vivo and demonstrates its importance in evading host innate immunity. In the absence of 2′-O-MTase activity, SARS-CoV-2 is rendered susceptible to the effector responses of IFIT1 and IFIT3 in combination. Importantly, such dependence of SARS-CoV-2 on the 2′-O-MTase function of NSP16 offers a novel target for future coronavirus antiviral drug development.
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/mutations-covid-19-study-shows-that-often-ignored-mutations-on-nsp4-and-nsp16-regions-of-sars-cov-2-genome-plays-critical-role-in-pathogenesis
https://www.thailandmedical.news/news/university-of-texas-study-discovers-that-sars-cov-2-utilizes-metal-dependent-mechanism-to-evade-immune-detection-in-the-human-host-body
https://www.thailandmedical.news/news/breaking-covid-19-genomics-sars-cov-2-has-mechanism-to-mimic-host-cellular-mrnas,-protecting-itself-from-host-innate-immune-restriction-
https://www.thailandmedical.news/news/breaking-coronavirus-news-contrary-to-beliefs-that-interferons-may-inhibit-sars-cov-2,-new-study-claims-that-ifns-actually-may-enhance-viral-invasio
https://www.thailandmedical.news/news/research-news-yale-researchers-claim-to-uncover-new-mechanism-of-immune-cell-activation
https://www.thailandmedical.news/news/breakthrough-german-study-discovers-that-sars-cov-2-vocs-dependent-on-ifitm2-for-replication-anti-ifitm2-antibody-inhibits-the-vocs-effectively
