COVID-19 News: Wuhan Study Finds That Host Protein ARF1 Is A Proviral Factor For SARS-Cov-2 Propagation
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 25, 2024 1 year, 2 months, 1 week, 1 day, 15 hours, 57 minutes ago
COVID-19 News: The ongoing global battle against the COVID-19 pandemic has spurred relentless research efforts to understand the intricate mechanisms of SARS-CoV-2 infection and to develop effective broad-spectrum antiviral therapies. In a recent study conducted by the Wuhan Institute of Virology in China, in collaboration with the University of Chinese Academy of Sciences, researchers have identified a host protein, ADP-ribosylation factor 1 (ARF1), as a key proviral factor responsible for bolstering the propagation of SARS-CoV-2 and its variants.
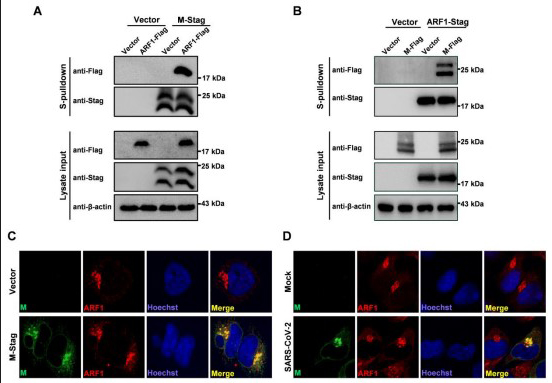 Interaction and colocalization of SARS-CoV-2 M and ARF1
(A)Validation of the ARF1 co-precipitation with SARS-CoV-2 M by pulldown and WB analyses. HEK293T cells were co-transfected with the plasmids encoding M fused with an S-tag (M-Stag) and Flag-tagged ARF1 (ARF1-Flag), or the corresponding control vectors. At 24 h post transfection, cells were lysed for S-pulldown assays, followed by WB analyses of the cell lysates (lysate input) and pulldown products with the indicated antibodies. (B) A reciprocal co-precipitation assay confirming the strong M-ARF1 interaction. HEK293T cells were co-transfected with the plasmids encoding ARF1-Stag and M-Flag, or the corresponding control vectors. At 24 h post transfection, cells were lysed for additional pulldown and WB analyses. (C) Colocalization of M with endogenous ARF1 in the context of transfection. HeLa cells were transfected with the plasmids expressing M-Stag or the control vector. Twenty-four hours later, cells were fixed for immunofluorescence assays (IFA) with the antibodies against Stag and ARF1, respectively. (D) Colocalization of M with endogenous ARF1 in the context of SARS-CoV-2 infection. HeLa cells were transfected with the ACE2 expression plasmid, followed by SARS-CoV-2 infection with a multiplicity of infection (MOI) of 1. At 24 h post infection (hpi), cells were fixed for IFA with the antibodies against M and ARF1.
Interaction and colocalization of SARS-CoV-2 M and ARF1
(A)Validation of the ARF1 co-precipitation with SARS-CoV-2 M by pulldown and WB analyses. HEK293T cells were co-transfected with the plasmids encoding M fused with an S-tag (M-Stag) and Flag-tagged ARF1 (ARF1-Flag), or the corresponding control vectors. At 24 h post transfection, cells were lysed for S-pulldown assays, followed by WB analyses of the cell lysates (lysate input) and pulldown products with the indicated antibodies. (B) A reciprocal co-precipitation assay confirming the strong M-ARF1 interaction. HEK293T cells were co-transfected with the plasmids encoding ARF1-Stag and M-Flag, or the corresponding control vectors. At 24 h post transfection, cells were lysed for additional pulldown and WB analyses. (C) Colocalization of M with endogenous ARF1 in the context of transfection. HeLa cells were transfected with the plasmids expressing M-Stag or the control vector. Twenty-four hours later, cells were fixed for immunofluorescence assays (IFA) with the antibodies against Stag and ARF1, respectively. (D) Colocalization of M with endogenous ARF1 in the context of SARS-CoV-2 infection. HeLa cells were transfected with the ACE2 expression plasmid, followed by SARS-CoV-2 infection with a multiplicity of infection (MOI) of 1. At 24 h post infection (hpi), cells were fixed for IFA with the antibodies against M and ARF1.
This discovery covered in this
COVID-19 News report not only sheds light on the fundamental biology of SARS-CoV-2 propagation but also unveils ARF1 as a promising therapeutic target with potential applications against evolving viral variants.
Evolutionary Threats of SARS-CoV-2 and the Need for Novel Antiviral Strategies
The relentless evolution of SARS-CoV-2, leading to the emergence of concerning variants such as Delta and Omicron, poses a persistent threat to public health. The continuous evolution of the virus has the potential to reduce the efficacy of existing vaccines and drugs, necessitating the development of innovative, broad-spectrum antiviral strategies. Despite extensive research on SARS-CoV-2, several aspects of its life cycle, including virion assembly, remain less understood. This study aims to bridge these knowledge gaps and explore host factors critical for viral assembly as potential targets for next-generation antiviral therapeutics.
The Structural Framework of SARS-CoV-2 and the Role of the M Protein
SARS-CoV-2 comprises four struc
tural proteins: spike (S), nucleocapsid (N), envelope (E), and membrane (M) proteins. Among these, the M protein plays a central role in orchestrating virion assembly and propagation. The virus assembles at the ER-Golgi intermediate compartment (ERGIC), where the M protein highly accumulates, creating a protein lattice that integrates other viral membrane proteins. Despite the significance of M in viral morphogenesis, the mechanisms behind its enrichment at the ERGIC have remained elusive.
Identification of ARF1 as a Proviral Factor
Through systematic functional experiments conducted both in vitro and in vivo, the researchers identified ARF1 as an M-interacting proviral factor crucial for the efficient propagation and pathogenicity of SARS-CoV-2 and its variants. Mechanistically, ARF1 facilitates the accumulation of M at the ERGIC, thereby promoting M-driven virion assembly and propagation. This groundbreaking finding highlights ARF1 as a key host factor involved in the viral life cycle and suggests its potential as a therapeutic target against SARS-CoV-2.
Disrupting ARF1-M Interaction: A Novel Antiviral Strategy
In pursuit of developing antiviral strategies, the researchers explored the disruption of the ARF1-M interaction as a potential therapeutic avenue. Small-molecule inhibitors targeting ARF1 were employed to disrupt the localization of both ARF1 and M at the ERGIC, leading to a significant inhibition of virion assembly and subsequent SARS-CoV-2 propagation. This approach demonstrated broad-spectrum antiviral potential, presenting a promising alternative to traditional viral component-targeting approaches.
Peptidomimetic Inhibition of ARF1-M Interaction: Proof of Concept
To further validate the significance of the ARF1-M interaction, the researchers designed a synthesized peptide mimicking the M-targeted motif of ARF1. This peptidomimetic inhibitor competitively blocked the M-ARF1 interaction, inhibiting M accumulation at the ERGIC and suppressing M-driven virion assembly. In vivo experiments using hamsters confirmed the therapeutic efficacy of this peptidomimetic inhibitor against SARS-CoV-2 infection and pathogenicity. These results provide proof of concept for the development of intervention strategies targeting the ARF1-M interaction interface.
Implications for Antiviral Therapeutics and Future Research
The study not only contributes critical insights into the basic biology of SARS-CoV-2 propagation but also unveils ARF1 as a candidate broad-spectrum therapeutic target. Antiviral interventions targeting host factors, such as ARF1, offer the potential for higher barriers to drug-resistance mutations and broader efficacy against evolving viral variants. As ARF1 is highly conserved across mammals, including humans, mice, and bats, targeting this host factor may not only offer therapeutic benefits for human patients but also serve as a basis for interspecies transmission control.
Conclusion
The identification of ARF1 as a proviral factor for SARS-CoV-2 and its variants opens new avenues for the development of antiviral therapeutics. By understanding the host-virus interactions critical for virion assembly, researchers have uncovered a potential weak point in the virus's life cycle. The disruption of the ARF1-M interaction, through small-molecule inhibitors or peptidomimetic strategies, presents promising prospects for the design of broad-spectrum anti-SARS-CoV-2 therapies. This study not only deepens our understanding of SARS-CoV-2 biology but also paves the way for innovative interventions against current and future viral challenges.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.researchsquare.com/article/rs-3812840/v1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
