COVID-19 Treatments: Spike Glycoprotein Promotes Hyper-Inflammatory Immune Responses Treatable By 4-Phenylburic Acid, Trametinib Or Ulixertinib
Source: COVID-19 Treatments Oct 06, 2020 5 years, 4 months, 5 days, 12 hours, 10 minutes ago
COVID-19 Treatments: Australian researchers from The University of Newcastle, Hunter Medical Research Institute-New South Wales, University of Technology Sydney and Chinese scientists from Jilin University-Changchun have in a new study confirmed that the SARS-CoV-2 spike glycoprotein subunit 1 can induce a pro-inflammatory signaling pathway (leading to cytokines deployment and epithelial damage in human bronchial epithelial cells). The study also uncovered drugs like 4-Phenylburic Acid, Trametinib Or Ulixertinib could help treat the conditions in infected human host.
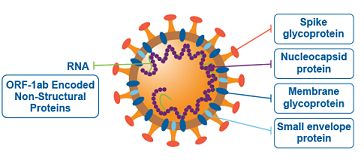
It has been found that the SARS-CoV-2 infection causes an inflammatory cytokine storm and acute lung injury. Currently there are no effective antiviral and/or anti-inflammatory therapies. (Dexamethasone has limited capabilities and can only be used in severe cases).
In this study the researchers demonstrated that the SARS-CoV-2 spike protein subunit 1 (CoV2-S1) induces high levels of NF-κB activations, production of pro-inflammatory cytokines and mild epithelial damage, in human bronchial epithelial cells. CoV2-S1-induced NF-κB activation requires S1 interaction with human ACE2 receptor and early activation of endoplasmic reticulum (ER) stress, and associated unfolded protein response (UPR), and MAP kinase signaling pathways.
The team developed an antagonistic peptide that inhibits S1-ACE2 interaction and CoV2-S1-induced productions of pro-inflammatory cytokines. The existing U.S.FDA-approved ER stress inhibitor, 4-phenylburic acid (4-PBA), and MAP kinase inhibitors, trametinib and ulixertinib, ameliorated CoV2-S1-induced inflammation and epithelial damage.
These study findings highlight the potentials of peptide-based antivirals for novel ACE2-utilising CoVs, while repurposing existing drugs may be used as treatments to dampen elevated inflammation and lung injury mediated by SARS-CoV-2.
The study findings are published on a preprint server and are pending peer-review.
https://www.biorxiv.org/content/10.1101/2020.09.30.317818v1
The SARS-CoV-2 coronavirus, the causative agent of the COVID-19) disease is especially prone to infecting human bronchial epithelial cells that support its replication. This prompts the innate immune response, which is critical in the early containment of the infection and subsequent viral spread.
It is already known that the spike glycoprotein of SARS-CoV-2 interacts with host angiotensin-converting enzyme 2 (ACE2) as a key initial step to viral replication. From the pathophysiological perspective, the receptor-binding domain (RBD) of subunit 1 (S1) represents the pivotal region for binding to the aforementioned receptor.
Subsequently this interaction kicks off heighten inflammatory response, which is primarily driven by early endoplasmic reticulum (ER) stress and its adaptive unfolded protein response (UPR), but also by activation of mitogen-activated protein (MAP) kinase signaling pathways.
Such early induction of ER-UPR results in activation of MAP kinase, and then both pathways synergistically lead to the activation of nuclear factor-κB (NF-κB) and production of pro-inflammatory cytokines such as interleukin-6, interleukin-1β, tumor necrosis facto
r-α (TNF-α) and C-C motif chemokine ligand 2 (CCL2) – the latter three contributing to acute lung injury.
To date, there are no specific antiviral or anti-inflammatory drugs available that can impact clinical outcomes in individuals with COVID-19.
The U.S.FDA EUA approved broad-spectrum antiviral drug remdesivir is an option for compassionate use against COVID-19 has shown dismal results.
More candidates that will hamper these specific inflammatory processes are desperately needed.
This need was of significant interest to the study team led by Dr Alan C-Y Hsu from the University of Newcastle and Hunter Medical Research Institute in New South Wales, Australia.
As SARS-CoV-2 S1 has been shown to induce NF-κB activation via its interaction with ACE2 and early activations of ER-UPR and MAP kinase signaling, these researchers designed a series of SARS-CoV-2-S1-antagonistic peptides and identified a peptide (designated AP-6) that successfully inhibits this interaction.
To assess whether SARS-CoV-2 spike glycoprotein induces the production of pro-inflammatory cytokines, they have used a minimally immortalized human bronchial epithelial cell line BCi-NS1.1, derived from human primary bronchial epithelial cells.
The key aim of the study was to assess if SARS-CoV-2 S1-mediated inflammation can be reduced by their SARS-CoV2-S1 inhibitory peptide AP-6, or with existing FDA-approved pharmacological inhibitors that target ER stress (4-phenylburic acid) or inhibit MAP kinase (trametinib and ulixertinib).
The study team had confirmed the notion that the SARS-CoV-2 S1 subunit and RBD instigate early ER-UPR and MAP kinase activations, subsequently leading to highly characteristic hyper-inflammatory response.
Dr Hsu told Thailand Medical News, "
Our data strong indicates that inflammation could be triggered by SARS-CoV-2 S1 even before viral replication occurs or in the absence of viral replication, As SARS-CoV-2 S1 is present throughout viral replication cycles and infection, our study data demonstrate that spike proteins are likely to be a major contributor to inflammation.”
Significantly, the study findings imply that triggered inflammatory storm and downstream consequences can be successfully inhibited by S1-inhibitory peptides, as well as existing FDA-approved ER stress inhibitor 4-phenylbuic acid and MAP kinase inhibitors trametinib and ulixertinib.
Dr Hsu added, "Collectively, SARS-CoV-2 S1 induced heightened production of inflammatory cytokines that are primarily driven by MAP kinase and ER stress cross-talks.”
The team also stressed that AP-6 truly demonstrates the feasibility of this proof-of-concept antiviral strategy specific for certain coronaviruses. This is rather important, because inflammatory injury resulting from the activation of the described inflammation cascade may be the first step to the acute respiratory distress syndrome (ARDS).
Hence, as antiviral drugs and vaccines are being developed and evaluated, existing FDA-approved ER stress and MAP kinase inhibitors could be deployed immediately in clinical trials as a potential treatment option for those with severe COVID-19.
For the time being, increased ER-UPR and MAP kinase activities as well as pro-inflammatory responses induced by CoV2-S1 could be substantially reduced by FDA-approved ER stress inhibitor 4-PBA and MAP kinase inhibitors trametinib and ulixertinib.
.jpg)
4-phenylburic acid or 4-PBA is a chemical chaperone currently used for treatment of urea cycle disorder, and has been used in clinical trials for diabetes, cystic fibrosis, sickle cell disease and neurodegenerative diseases (Mimori et al., 2012).
https://pubmed.ncbi.nlm.nih.gov/21612962/
https://pubmed.ncbi.nlm.nih.gov/16931765/
https://pubmed.ncbi.nlm.nih.gov/7528572/ https://pubmed.ncbi.nlm.nih.gov/22223342/
Trametinib is a MAP kinase inhibitor used for melanoma, and ulixertinib is a highly potent, selective, reversible, ERK1/2 inhibitor used as cancer treatment. Re-purposing these drugs that has a well-documented safety profile in humans could expedite rapid deployment of these drugs for severe COVID-19.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6570520/
https://pubmed.ncbi.nlm.nih.gov/29247021/
For more on
COVID-19 Treatments, keep on logging to Thailand Medical News.

.jpg)