Sebastian Lavoie Fact checked by:Thailand Medical News Team Sep 07, 2024 7 months, 1 week, 6 days, 17 hours, 26 minutes ago
Vaccine News: Researchers from the Istituto Dermopatico dell’Immacolata (IDI)-IRCCS, Rome, Italy, conducted a study to explore the relationship between COVID-19 vaccinations and the onset of bullous pemphigoid (BP), an autoimmune blistering disease commonly affecting the elderly. Their findings offer an intriguing look into how the vaccine could be a potential factor in accelerating the onset of this condition in predisposed individuals.
 COVID-19 vaccines can increase the risk of the onset of Bullous Pemphigoid
What is Bullous Pemphigoid?
COVID-19 vaccines can increase the risk of the onset of Bullous Pemphigoid
What is Bullous Pemphigoid?
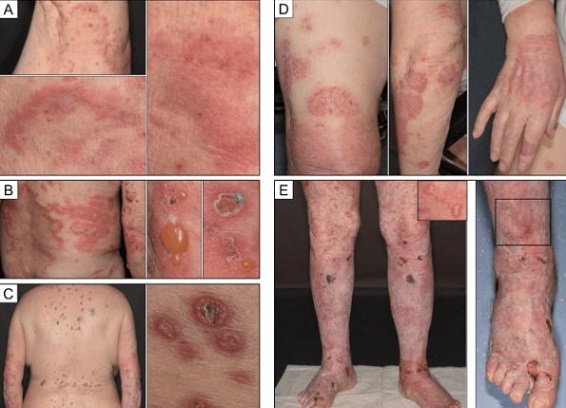
Bullous pemphigoid (BP) is a chronic autoimmune disease characterized by the formation of large, fluid-filled blisters on the skin. These blisters result from the immune system mistakenly attacking the skin's basement membrane, specifically targeting the BP180 and BP230 proteins. The disease is most common among elderly individuals and is often triggered by medications, infections, or other underlying health conditions.
COVID-19 Vaccines and Bullous Pemphigoid: A Growing Concern
Since the start of mass COVID-19 vaccinations, there has been increasing interest in potential vaccine-related autoimmune reactions. More than 90 cases of BP linked to COVID-19 vaccines have been reported worldwide. This
Vaccine News report focuses on a study that sought to investigate the connection between BP onset and COVID-19 vaccinations, shedding light on whether the vaccine could accelerate the onset of the disease in predisposed individuals.
The study enrolled 64 BP patients, 14 of whom developed the disease shortly after receiving the COVID-19 vaccine. These patients were referred to as vaccine-associated (VA) cases, while the remaining 50 were classified as vaccine-non-associated (VNA). The research team analyzed clinical data, immunological profiles, and the timing of BP onset to better understand the potential role of the vaccine in triggering the condition.
Key Study Findings
One of the most notable findings of the study was that 22% of the patients experienced BP onset within five weeks of receiving their COVID-19 vaccine. These patients were predominantly elderly, with a mean age of 76.7 years for VA patients and 80 years for VNA patients. Interestingly, the study found no significant difference in age, gender, or clinical presentation between the two groups, indicating that the vaccine itself did not lead to more severe or unique symptoms of Bullous Pemphigoid.
In terms of vaccines, the majority of VA patients received the Pfizer-BioNTech vaccine (71.4%), while a smaller percentage received the Moderna mRNA-1273 (21.4%) or AstraZeneca vaccines (7.1%). However, there was no statistically significant difference in the type of vaccine received between VA and VNA patients, suggesting that the risk of developing Bullous Pemphigoid may not be tied to a specific vaccine brand.
Both groups showed similar immune responses, with no major differences in the levels of autoantibodies (anti-BP180 and anti-BP230) that are t
ypically associated with Bullous Pemphigoid. However, there were slight differences in reactivity to certain epitopes of the BP180 protein, with VA patients showing higher levels of certain autoantibodies. This suggests that while the COVID-19 vaccine may not directly cause BP, it could act as a trigger that accelerates the onset of the disease in individuals who already have subclinical autoimmunity.
The monthly distribution of Bullous Pemphigoid onset during the vaccination campaign also raised some interesting observations. The study found a significant increase in BP cases in April and May 2021, which coincided with the peak of COVID-19 vaccinations in Italy. This temporal association between vaccine administration and BP onset suggests that the vaccine may have played a role in triggering the disease in some cases. However, the study was careful to note that this does not establish a causal relationship, and further research is needed to fully understand the mechanisms involved.
The Role of Predisposing Factors
While the COVID-19 vaccine may be a potential trigger for Bullous Pemphigoid, the study emphasizes that it is likely not the sole factor responsible for the onset of the disease. Genetic predisposition, underlying health conditions, and the use of certain medications (such as diuretics and inhalers) may all contribute to the development of BP. In fact, the study found that VNA patients were more likely to use diuretics, while VA patients had higher rates of inhaler use, suggesting that these medications could play a role in triggering the disease in certain individuals.
Conclusion
In conclusion, the study provides valuable insights into the potential link between COVID-19 vaccines and Bullous Pemphigoid onset. While the vaccine may act as a trigger in predisposed individuals, it is unlikely to be the sole cause of the disease. The findings highlight the need for further research to explore the mechanisms behind vaccine-associated BP and to identify individuals who may be at higher risk of developing the condition.
The study findings were published in the peer-reviewed journal: Vaccines.
https://www.mdpi.com/2076-393X/12/9/1016
For the latest
Vaccine News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/peer-reviewed-study-warns-that-covid-19-vaccines-are-causing-endotheliopathy-or-glycocalyx-degradation
https://www.thailandmedical.news/news/indian-researchers-developing-new-edible-probiotic-yoghurt-based-vaccine-for-covid-19
https://www.thailandmedical.news/news/chinese-researchers-warn-that-current-vaccines-are-inefficient-in-protecting-against-emerging-mpox-virus
