Cullin 5 (CUL5) Complex Offers New Hope for COVID-19 Treatment by Disabling ORF9b Proteins
Nikhil Prasad Fact checked by:Thailand Medical News Team Jun 28, 2024 9 months, 4 weeks, 9 hours, 37 minutes ago
COVID-19 News: Since the COVID-19 pandemic began, scientists worldwide have been working tirelessly to understand SARS-CoV-2, the virus that causes the disease. This virus is particularly skilled at infecting people and evading their immune systems, making it a formidable opponent. One of the reasons for its success is a small but crucial protein called ORF9b. This protein helps the virus hide from our immune defenses, allowing it to replicate and spread. This
COVID-19 News report covers a study by researchers from Central South University, Changsha-China that unravels the role of Cullin 5 (CUL5) Complex and other proteins such as TOM70 and HSP90α in COVID-19 pathogenesis and how targeting the viral protein ORF9b could help in the fight against COVID-19.
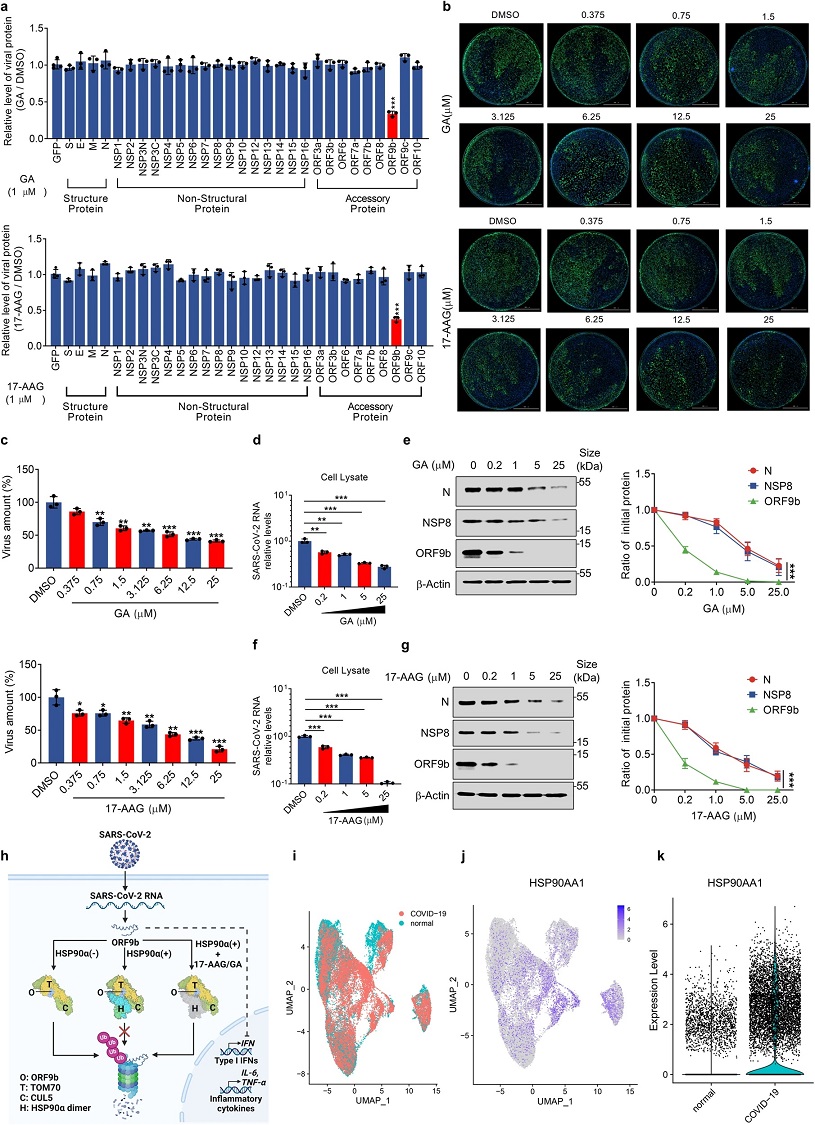 HSP90 inhibitors restrict the replication of SARS-CoV-2 by promoting ORF9b degradation. a The plasmids containing Strep-tagged SARS-CoV-2 viral genes were transfected into HEK293T cells and treated with indicated concentrations of GA or 17-AAG for 24 h. The cells were lysed to detect the viral protein levels and quantification of different viral proteins was normalized to β-actin. b and c Calu3 cells treated with indicated increasing concentrations of GA (b-up), 17-AAG (b-down) or DMSO were inoculated with SARS-CoV-2 at MOI = 1 for 24 h. Quantification of relative virus amount was measured by immunofluorescence (c). d–g Calu3 cells infected with SARS-CoV-2 at a MOI = 1 were treated with indicated concentrations of GA (d and e) or 17-AAG (f and g) for 24 h. Relative mRNA (left) and protein levels (right) were detected by qRT-PCR and Western blot as indicated. h A schematic diagram illustrates the regulation of the TOM70–CUL5–HSP90α complex on ORF9b. The interaction model of the complex was based on a predicted model generated using AlphaFold, as shown in Fig. 5l. Different components are represented by distinct colors: ORF9b is depicted in sky blue, TOM70 in yellow, CUL5 in yellow-green, HSP90α monomer 1 in cyan, HSP90α monomer 2 in aquamarine, and the nonfunctional HSP90α dimer in gray. This diagram demonstrates how, upon SARS-CoV-2 entry, host cells counteract viral immune evasion by targeting ORF9b for ubiquitination and subsequent degradation. In the absence of HSP90α, TOM70 and CUL5 mediate ORF9b degradation via the ubiquitin-proteasome pathway (left). Conversely, in the presence of HSP90α, ORF9b is shielded from degradation (middle). However, HSP90 inhibitors such as 17-AAG/GA deactivate HSP90αα, leading to the degradation of ORF9b (right). i–k A dataset was obtained from GEO with accession number GSE171524 to analyze the difference in RNA levels of HSP90AA1 between lung epithelial cells of COVID-19 patients and healthy control. Group origins of cells (i) and RNA levels of HSP90AA1 in single cells (j) were shown with a UMAP plot. Violin plot (k) was performed to show the difference in HSP90AA1 RNA levels between lung epithelial cells of COVID-19 patients and
healthy control. Quantification was shown as mean ± s.d. n = 3 independent experiments.
The Deceptive ORF9b Protein
HSP90 inhibitors restrict the replication of SARS-CoV-2 by promoting ORF9b degradation. a The plasmids containing Strep-tagged SARS-CoV-2 viral genes were transfected into HEK293T cells and treated with indicated concentrations of GA or 17-AAG for 24 h. The cells were lysed to detect the viral protein levels and quantification of different viral proteins was normalized to β-actin. b and c Calu3 cells treated with indicated increasing concentrations of GA (b-up), 17-AAG (b-down) or DMSO were inoculated with SARS-CoV-2 at MOI = 1 for 24 h. Quantification of relative virus amount was measured by immunofluorescence (c). d–g Calu3 cells infected with SARS-CoV-2 at a MOI = 1 were treated with indicated concentrations of GA (d and e) or 17-AAG (f and g) for 24 h. Relative mRNA (left) and protein levels (right) were detected by qRT-PCR and Western blot as indicated. h A schematic diagram illustrates the regulation of the TOM70–CUL5–HSP90α complex on ORF9b. The interaction model of the complex was based on a predicted model generated using AlphaFold, as shown in Fig. 5l. Different components are represented by distinct colors: ORF9b is depicted in sky blue, TOM70 in yellow, CUL5 in yellow-green, HSP90α monomer 1 in cyan, HSP90α monomer 2 in aquamarine, and the nonfunctional HSP90α dimer in gray. This diagram demonstrates how, upon SARS-CoV-2 entry, host cells counteract viral immune evasion by targeting ORF9b for ubiquitination and subsequent degradation. In the absence of HSP90α, TOM70 and CUL5 mediate ORF9b degradation via the ubiquitin-proteasome pathway (left). Conversely, in the presence of HSP90α, ORF9b is shielded from degradation (middle). However, HSP90 inhibitors such as 17-AAG/GA deactivate HSP90αα, leading to the degradation of ORF9b (right). i–k A dataset was obtained from GEO with accession number GSE171524 to analyze the difference in RNA levels of HSP90AA1 between lung epithelial cells of COVID-19 patients and healthy control. Group origins of cells (i) and RNA levels of HSP90AA1 in single cells (j) were shown with a UMAP plot. Violin plot (k) was performed to show the difference in HSP90AA1 RNA levels between lung epithelial cells of COVID-19 patients and
healthy control. Quantification was shown as mean ± s.d. n = 3 independent experiments.
The Deceptive ORF9b Protein
The ORF9b protein is produced by the virus and is adept at stopping our body's production of interferon, a key component of our immune response that fights off viral infections. This makes ORF9b a vital player in the virus's strategy to establish and maintain infection. By suppressing interferon, ORF9b helps the virus go unnoticed by our immune system, making it harder for our bodies to mount an effective defense.
Discovering ORF9b's Achilles' Heel
Recent research has shed light on a significant vulnerability in ORF9b. Scientists have discovered that our cells can tag ORF9b for destruction through a process called ubiquitination. This process involves attaching a small molecule called ubiquitin to ORF9b, marking it for degradation by the cell's proteasome - a kind of cellular recycling bin. This tagging and destruction process is a critical part of our body's natural defense mechanism against viruses.
The Cullin 5 Complex: The Body's Natural Defense
At the heart of this defense mechanism is a protein complex known as Cullin 5 (CUL5). CUL5 works in conjunction with other proteins, including TOM70 and HSP90α, to tag ORF9b for destruction. In this complex, CUL5 acts as a scaffold, providing a structure that brings together the necessary components to attach ubiquitin to ORF9b. TOM70 acts as a substrate receptor, helping to identify and bind ORF9b, while HSP90α stabilizes the complex.
How the Virus Fights Back
However, the virus has its own countermeasures. It uses HSP90α, a heat shock protein, to protect ORF9b from being tagged and destroyed. HSP90α stabilizes ORF9b, preventing it from being degraded by the ubiquitination process. This allows ORF9b to continue its role in helping the virus evade the immune system, making it harder for our bodies to fight off the infection.
A New Hope: HSP90 Inhibitors
Researchers have found a way to tip the balance in favor of our immune system. By using drugs that inhibit HSP90α, such as geldanamycin (GA) and 17-AAG, they can disrupt the stabilization of ORF9b. These inhibitors prevent HSP90α from protecting ORF9b, allowing CUL5 to tag it for destruction. This results in the rapid degradation of ORF9b, significantly reducing the virus's ability to replicate and spread.
Evidence from COVID-19 Patients
Further studies on lung cells from COVID-19 patients have shown that levels of HSP90α are significantly higher in these patients compared to healthy individuals. This suggests that the virus increases the production of HSP90α to protect ORF9b, enabling it to evade the immune response more effectively. Targeting HSP90α with inhibitors could, therefore, be a powerful strategy to help our bodies clear the virus.
Laboratory Experiments Show Promise
In laboratory experiments, cells treated with HSP90 inhibitors exhibited reduced levels of ORF9b. These treated cells were better able to produce antiviral signals, such as interferon, enhancing their ability to fight off the virus. This provides strong evidence that HSP90 inhibitors could be an effective treatment for COVID-19, helping to bolster the body's natural defenses.
Success in Animal Models
To confirm these findings, researchers used a mouse model of COVID-19. Mice treated with HSP90 inhibitors showed significantly lower viral loads and less severe disease symptoms compared to untreated mice. This suggests that these drugs could help reduce the severity of COVID-19 in humans, providing a new tool in the fight against the virus.
The Host-Virus Battle: A Constant Struggle
The interaction between ORF9b and our immune system highlights the ongoing battle between host and virus. While the virus uses ORF9b to suppress our defenses, our cells use CUL5 to fight back. HSP90α plays a crucial role in this battle, protecting ORF9b unless we use drugs to inhibit it. This discovery underscores the complexity of the host-virus interaction and the potential for new therapeutic strategies.
Implications for Future Treatments
This discovery opens up exciting new possibilities for treating COVID-19. By using HSP90 inhibitors, we could potentially improve the immune response in COVID-19 patients, helping their bodies to clear the virus more effectively. This strategy could be particularly important for those with underlying conditions that make them more vulnerable to severe disease.
Conclusion: A New Weapon in the Fight Against COVID-19
The fight against COVID-19 is far from over, but discoveries like this bring new hope. Understanding the role of ORF9b and how our bodies can destroy it with the help of CUL5 and HSP90 inhibitors provides a promising strategy for new treatments. This research highlights the intricate dance between virus and host and offers a new target in our battle against this global pandemic. By targeting the virus's ability to hide from our immune system, we can develop more effective treatments and move closer to ending the pandemic.
Looking Forward
The next steps in this research involve clinical trials to test the safety and efficacy of HSP90 inhibitors in COVID-19 patients. If successful, these drugs could become a crucial part of the arsenal against COVID-19, helping to reduce the severity of the disease and save lives. As we continue to learn more about the virus and how it interacts with our bodies, we can develop better strategies to combat it and protect public health.
Final Thoughts
In summary, the discovery of the role of the CUL5-TOM70-HSP90α complex in regulating the stability of the ORF9b protein opens up new avenues for treating COVID-19. By inhibiting HSP90α, we can enhance the degradation of ORF9b, making it harder for the virus to evade our immune system. This innovative approach highlights the potential for targeting viral proteins and their interactions with host proteins as a strategy for developing new antiviral therapies. As research progresses, we remain hopeful that these findings will lead to effective treatments and help bring an end to the COVID-19 pandemic.
The study findings were published in the peer reviewed journal: Signal Transduction and Targeted Therapy.
https://www.nature.com/articles/s41392-024-01874-5
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-accessory-proteins-orf6,-orf8,-orf9b,-orf9c-have-the-ability-to-trigger-inflammatory-and-profibrotic-processes-through-il11-signaling
https://www.thailandmedical.news/news/shandong-university-study-uncovers-that-sars-cov-2-orf9b-antagonizes-type-i-and-iii-interferons-by-targeting-components-of-signaling-pathways
https://www.thailandmedical.news/news/breaking-covid-19-research-coronavirus-study-reveals-that-sars-cov-2-orf9b-protein-binds-to-human-host-mitochondria-protein-tom70
