Decoding Long COVID-19: How Interferon-Lambda Modulates T Lymphocyte PD-1 Via The AKT/GSK3Beta Pathway
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 02, 2024 1 year, 9 months, 3 weeks, 8 hours, 42 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has affected millions of lives worldwide. While the acute phase of the disease primarily manifests as respiratory illness, a growing body of evidence suggests that a significant proportion of individuals experience lingering symptoms and complications long after the initial infection has resolved. This phenomenon, known as "long COVID-19," presents a unique challenge to clinicians and researchers alike, as the underlying mechanisms are still not fully understood. In this
COVID-19 News report, we delve into the intricate interplay between T lymphocyte responses, cytokine signaling, and immune checkpoint regulation to shed light on the immunological complexities of long COVID-19.
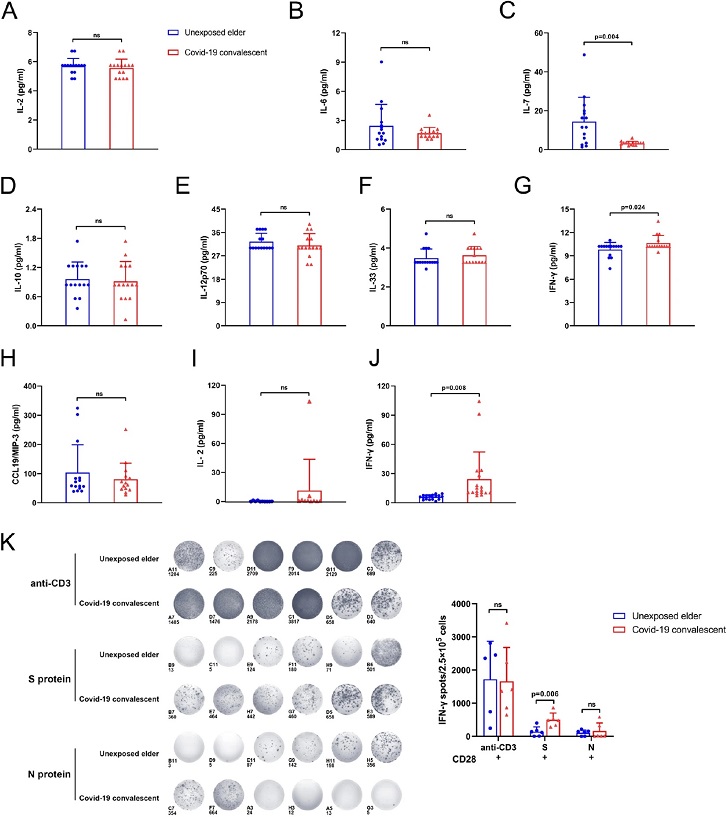 IFN-γ was increased in the plasma from COVID-19 convalescent patients. Luminex and ELISA detection of unexposed elderly individuals and COVID-19 convalescent plasma cytokines. (A–H) is Luminex detection of cytokines related to PD-1 in the elder group and COVID-19 convalescent plasma, including IL-2, IL-6, IL-7, IL-10, IL-12p70, IL-33, IFN-γ, CCL19/MIP-3. The bar chart shows that, compared with unexposed elderly individuals, COVID-19 convalescent inflammatory factor IFN-γ secretion increased, while IL-7 secretion decreased, and there was a significant difference. There was no significant difference in the rest (n = 15). (I,J) ELISA was used to compare the expression of IL-2 and IFN-γ in the plasma of the two groups [(I) n = 10; (J) n = 18]. Figures (G) and (J) show two different methods for detecting the amount of IFN-γ in plasma, and the results of the two methods are consistent. (K) Unexposed elderly and COVID-19 convalescent PBMCs under anti-CD3/CD28, S protein and N protein stimulation. ELISPOT detected the secretion of IFN-γ. Cells were seeded at a density of 2 × 105 cells/well, and the activation time was 48 h. Statistical analysis of the number of spots in different ELISPOT wells shows that under S protein stimulation conditions, COVID-19 convalescent T cells can secrete more IFN-γ (n = 6).
Immune Dysregulation in COVID-19
IFN-γ was increased in the plasma from COVID-19 convalescent patients. Luminex and ELISA detection of unexposed elderly individuals and COVID-19 convalescent plasma cytokines. (A–H) is Luminex detection of cytokines related to PD-1 in the elder group and COVID-19 convalescent plasma, including IL-2, IL-6, IL-7, IL-10, IL-12p70, IL-33, IFN-γ, CCL19/MIP-3. The bar chart shows that, compared with unexposed elderly individuals, COVID-19 convalescent inflammatory factor IFN-γ secretion increased, while IL-7 secretion decreased, and there was a significant difference. There was no significant difference in the rest (n = 15). (I,J) ELISA was used to compare the expression of IL-2 and IFN-γ in the plasma of the two groups [(I) n = 10; (J) n = 18]. Figures (G) and (J) show two different methods for detecting the amount of IFN-γ in plasma, and the results of the two methods are consistent. (K) Unexposed elderly and COVID-19 convalescent PBMCs under anti-CD3/CD28, S protein and N protein stimulation. ELISPOT detected the secretion of IFN-γ. Cells were seeded at a density of 2 × 105 cells/well, and the activation time was 48 h. Statistical analysis of the number of spots in different ELISPOT wells shows that under S protein stimulation conditions, COVID-19 convalescent T cells can secrete more IFN-γ (n = 6).
Immune Dysregulation in COVID-19
Severe cases of COVID-19 are characterized by dysregulated immune responses, marked by an exaggerated release of proinflammatory cytokines, often referred to as a cytokine storm. This hyperinflammatory state can lead to tissue damage and multiorgan dysfunction, contributing to the severity of the disease. Furthermore, T lymphocyte exhaustion and dysfunction have been implicated in the pathogenesis of severe COVID-19, with CD8+ T cells, in particular, playing a crucial role in viral clearance. However, persistent activation and dysregulation of T lymphocytes in convalescent COVID-19 patients suggest ongoing immune perturbations beyond the acute phase of the disease.
t;
Role of PD-1 in Immune Dysfunction
Central to the regulation of T cell responses is the programmed death receptor 1 (PD-1), an inhibitory receptor expressed on activated T cells. PD-1 engagement with its ligands, PD-L1 and PD-L2, inhibits T cell activation and effector functions, thereby maintaining immune homeostasis and preventing autoimmunity. However, sustained PD-1 expression on T cells, often observed in chronic infections and cancer, can lead to T cell exhaustion and impaired antiviral immunity. In the context of COVID-19, elevated PD-1 expression on T lymphocytes has been associated with disease severity and poor clinical outcomes, suggesting a potential role for PD-1-mediated immune dysfunction in COVID-19 pathogenesis.
Cytokine Storms and IFN-γ in COVID-19
The dysregulated immune response in COVID-19 is characterized by the overproduction of proinflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). Among these cytokines, IFN-γ plays a critical role in modulating immune responses to viral infections. IFN-γ exerts pleiotropic effects on various immune cells, including T lymphocytes, macrophages, and natural killer (NK) cells, promoting antiviral defense mechanisms. However, dysregulated IFN-γ production has been implicated in the pathogenesis of cytokine storms and tissue damage in severe COVID-19 cases. Moreover, recent studies suggest that IFN-γ may play a dual role in regulating PD-1 expression on T cells, highlighting its complex involvement in COVID-19 immunopathogenesis.
Study Overview
To elucidate the immunological mechanisms underlying long COVID-19, researchers from Jiangsu Province People’s Hospital, Nanjing Medical University First Affiliated Hospital, and Xuzhou Municipal Hospital conducted a comprehensive study focusing on T lymphocyte responses and cytokine signaling in convalescent COVID-19 patients. The study aimed to investigate the relationship between IFN-γ, PD-1 expression, and the AKT/GSK3β signaling pathway in T lymphocytes, shedding light on potential therapeutic targets for managing long COVID-19.
Key Findings
The study recruited a cohort of convalescent COVID-19 patients and analyzed their immune profiles compared to age-matched unexposed individuals. Interestingly, convalescent patients exhibited decreased PD-1 expression on CD8+ T lymphocytes, alongside elevated plasma levels of IFN-γ. These findings suggest a potential link between IFN-γ signaling and PD-1 regulation in T cells post-COVID-19 recovery. Further experiments revealed that IFN-γ played a crucial role in suppressing PD-1 expression on CD8+ T cells, potentially contributing to immune dysregulation in long COVID-19.
Mechanistic Insights
Bioinformatics analysis identified the AKT/GSK3β signaling pathway as a potential regulator of the IFN-γ/PD-1 axis in CD8+ T cells from convalescent COVID-19 patients. Experimental validation demonstrated that IFN-γ reduced PD-1 expression via the AKT/GSK3β pathway. Moreover, modulation of AKT and GSK3β activity affected PD-1 expression on CD8+ T cells, highlighting the intricate interplay between cytokine signaling and immune checkpoint regulation. These mechanistic insights provide valuable information for understanding the pathogenesis of long COVID-19 and identifying potential therapeutic targets for intervention.
Implications for Long COVID-19 Management
The findings from this study have important implications for the management of long COVID-19. Targeting the AKT/GSK3β pathway or modulating IFN-γ levels could offer new therapeutic strategies for mitigating immune dysregulation and alleviating long-term complications associated with COVID-19 recovery. Additionally, the identification of specific immune markers, such as PD-1 expression on T lymphocytes, could aid in the clinical monitoring and management of convalescent COVID-19 patients, facilitating early intervention and personalized treatment approaches.
Conclusion
In conclusion, the study provides valuable insights into the immunological dynamics of long COVID-19, highlighting the intricate interplay between T lymphocyte responses, cytokine signaling, and immune checkpoint regulation. The identification of the IFN-γ/PD-1 axis and the AKT/GSK3β signaling pathway as key regulators of immune dysregulation post-COVID-19 recovery offers new avenues for therapeutic intervention and personalized management strategies. Further research is warranted to validate these findings and translate them into clinical practice, ultimately improving outcomes for individuals affected by long COVID-19.
The study findings were published in the peer reviewed journal: Scientific Reports.
https://link.springer.com/article/10.1038/s41598-024-55191-6
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
