Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 02, 2024 1 year, 2 weeks, 5 days, 8 hours, 48 minutes ago
Medical News: Cancer arises when cells lose control over their growth and replication, often because of accumulated genetic mutations. One of the most critical proteins in our body that safeguards the genome from such damage is p53, commonly referred to as the "guardian of the genome." This powerful tumor suppressor plays a key role in detecting and responding to DNA damage, repairing it, or eliminating cells if the damage is beyond repair. A groundbreaking study conducted by researchers from Xinjiang University’s School of Pharmaceutical Science and the Xinjiang Key Laboratory of Biological Resources and Genetic Engineering in China, sheds light on the intricate workings of p53, how its mutations contribute to cancer, and its potential as a target for innovative therapies.
 Decoding the Role of p53 in Cancer
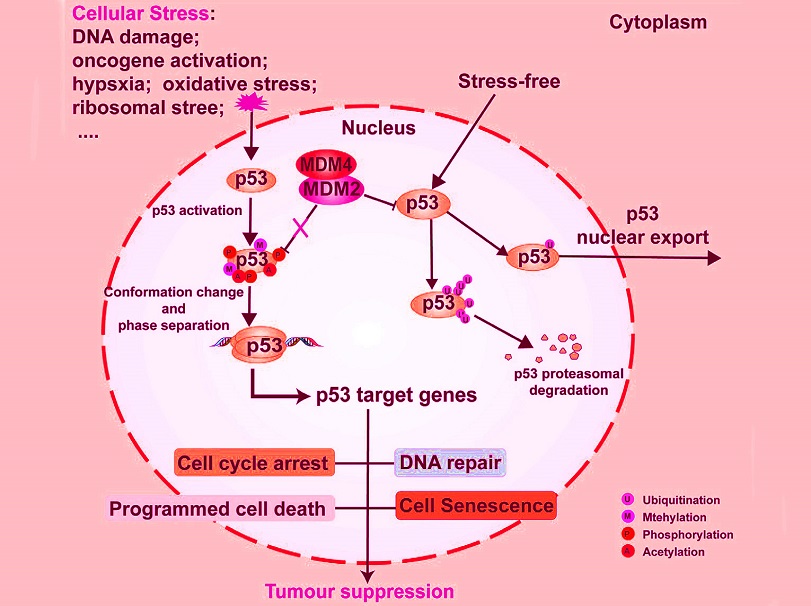
Regulation of p53 expression. (1) MDM2/MDM4 play a role in maintaining p53 homeostasis under non-stress conditions by regulating p53 ubiquitination. (2) MDM2/MDM4-mediated p53 monoubiquitination facilitates its nuclear export. The MDM2/MDM4-mediated polyubiquitination of p53 facilitates its proteasomal degradation. (3) The phosphorylation of p53 has been demonstrated to antagonize the interaction between MDM2/MDM4 and p53, thereby mediating the accumulation and activation of p53 protein levels. (4) The methylation and acetylation of p53 regulate its transcriptional activity and target specificity towards DNA.
How p53 Keeps Cells Healthy
Decoding the Role of p53 in Cancer
Regulation of p53 expression. (1) MDM2/MDM4 play a role in maintaining p53 homeostasis under non-stress conditions by regulating p53 ubiquitination. (2) MDM2/MDM4-mediated p53 monoubiquitination facilitates its nuclear export. The MDM2/MDM4-mediated polyubiquitination of p53 facilitates its proteasomal degradation. (3) The phosphorylation of p53 has been demonstrated to antagonize the interaction between MDM2/MDM4 and p53, thereby mediating the accumulation and activation of p53 protein levels. (4) The methylation and acetylation of p53 regulate its transcriptional activity and target specificity towards DNA.
How p53 Keeps Cells Healthy
The cells in our body constantly face threats from internal metabolic by-products and external environmental factors such as radiation and chemicals. These threats can cause damage to DNA, leading to mutations. When left unchecked, such mutations can result in uncontrolled cell growth, the hallmark of cancer. Fortunately, p53 is equipped to combat these dangers.
When DNA damage occurs, p53 is activated through a series of molecular signals. This activation leads to three critical outcomes:
-Cell Cycle Arrest: p53 halts the cell cycle, giving the cell enough time to repair the DNA damage. It achieves this by activating a gene called p21, which inhibits cell cycle progression.
-DNA Repair: p53 directly interacts with and promotes the activity of DNA repair pathways, such as nucleotide excision repair and base excision repair. This ensures the integrity of the cell's genetic material is maintained.
-Apoptosis: If the DNA damage is too severe to be fixed, p53 initiates a controlled cell death process called apoptosis. This eliminates potentially dangerous cells and prevents them from turning into cancer.
This
Medical News report highlights the remarkable precision of p53 in maintaining cellular stability, noting how it activates genes involved in repair, halts cell division, and eliminates threats.
The Tragic Twist: When p53 Goes Wrong
Despite its critical
protective role, p53 is not immune to malfunction. The gene encoding p53, TP53, is the most frequently mutated gene in human cancers, found in approximately 50% of all tumors. These mutations render p53 unable to perform its protective functions. Worse, certain mutations in p53 not only eliminate its tumor-suppressing abilities but also give it new cancer-promoting functions.
The researchers identified two key effects of mutated p53:
-Loss of Function (LOF): Mutant p53 loses its ability to regulate the cell cycle, repair DNA, and trigger apoptosis. This allows cells with damaged DNA to continue dividing, leading to the accumulation of mutations and eventual tumor formation.
-Gain of Function (GOF): Some p53 mutations endow the protein with oncogenic properties, such as reprogramming cellular metabolism to favor cancer growth, promoting angiogenesis (the formation of blood vessels to supply tumors), and suppressing the immune response.
Mutant p53 can also interfere with normal p53 activity by forming dysfunctional complexes with the remaining functional protein, a phenomenon known as the dominant-negative effect. This dual role of p53 mutations in cancer progression makes it a challenging but compelling target for therapy.
Therapeutic Approaches Targeting p53
The researchers from Xinjiang University outlined several innovative approaches to harness the therapeutic potential of p53 in cancer treatment. These approaches aim to restore p53’s normal function or counteract the effects of its mutations.
-Restoring Wild-Type p53: Small molecules such as APR-246 have shown promise in reactivating mutant p53 by restoring its wild-type structure. These drugs bind to mutant p53 and reestablish its ability to bind DNA, enabling it to resume its tumor-suppressing functions.
-Targeting Negative Regulators: Proteins like MDM2 and MDM4, which normally suppress p53 to regulate its activity, can become overactive in cancers. Drugs that inhibit these proteins can release p53 from suppression, allowing it to function effectively.
-Immunotherapy: Researchers are exploring p53-based vaccines and antibodies that train the immune system to recognize and destroy cancer cells harboring mutant p53.
-Gene Therapy: Techniques to deliver functional TP53 genes to cancer cells or to edit the defective genes are in development, offering a way to directly address the root cause of p53 dysfunction.
Study Findings: A New Frontier in Cancer Treatment
The study revealed exciting possibilities for targeting p53 pathways in cancer therapy. For instance, mutant p53 proteins that exhibit gain-of-function properties can be targeted with drugs that degrade these harmful proteins, while therapies aimed at restoring p53 function are particularly effective in cancers with loss-of-function mutations.
One promising drug, APR-246, works by converting mutant p53 back to its wild-type form. Another, COTI-2, not only restores mutant p53’s function but also affects cancer cells through p53-independent pathways, enhancing its versatility as a therapeutic agent. Immunotherapies targeting p53-derived peptides are also in clinical trials, offering hope for harnessing the immune system against p53-deficient cancers.
The Role of MDM2 and MDM4 in p53 Regulation
The study also highlighted the critical role of MDM2 and MDM4, two proteins that regulate p53. These proteins tag p53 for degradation under normal conditions to prevent unnecessary cell death. However, in cancer, their overexpression can suppress p53’s activity, even when it is functional. Drugs that block MDM2/MDM4 can help restore p53’s tumor-suppressing abilities.
Challenges and Future Directions
While the therapeutic potential of targeting p53 is immense, there are challenges. The diversity of p53 mutations, tumor heterogeneity, and the need to avoid damage to normal cells make it difficult to develop universal treatments. Additionally, reactivating mutant p53 or targeting its pathways requires precise delivery methods to ensure efficacy and minimize side effects.
Despite these challenges, the study underscores that p53 remains one of the most promising targets in cancer therapy. By combining molecular biology, drug development, and precision medicine, researchers are optimistic about turning p53-targeted therapies into viable treatment options.
Conclusions
The study findings emphasize the central role of p53 in cancer prevention and the promise of targeting its pathways in therapy. As a master regulator of cellular health, p53’s ability to repair DNA, halt cell division, or trigger cell death makes it an invaluable tool in the fight against cancer. However, its frequent mutation in tumors poses both a challenge and an opportunity for researchers.
Restoring or mimicking p53’s function could revolutionize cancer treatment, offering new hope to patients with cancers that currently lack effective therapies. While challenges remain, the progress in understanding p53’s biology and developing targeted therapies is a testament to the potential of modern science to combat cancer at its genetic roots.
The study findings were published in the peer-reviewed International Journal of Molecular Sciences.
https://www.mdpi.com/1422-0067/25/23/12928
For the latest Cancer News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-spike-suppresses-p53-dependent-gene-activation-impacting-tumorigenesis-tumor-progression-and-chemotherapy-sensitivity
https://www.thailandmedical.news/news/spanish-study-discovers-that-sars-cov-2-infections-leads-to-downregulation-of-p53-a-critical-cancer-protective-gene
https://www.thailandmedical.news/news/great-news-study-shows-that-sars-cov-2-protein-nsp13-is-able-to-cause-dna-damage-and-dysregulate-tumor-suppressor-gene-p53,-increasing-cancer-risk
https://www.thailandmedical.news/news/covid-19-research-bioinformatics-study-reveals-p53-signaling,-genes-nfkbia,-c3,-ccl20-are-among-cellular-pathways-and-genes-affected-by-coronavirus
https://www.thailandmedical.news/news/men-more-prone-to-cancer-due-to-tp53-mutations-on-x-chromosome
https://www.thailandmedical.news/news/p53-discovered-to-have-dual-roles-in-not-only-fighting-tumors-but-also-promoting-growth-in-certain-cancers-
https://www.thailandmedical.news/news/sars-cov-2-rna-replication-is-enhanced-by-p53-independent-g1-cell-cycle-arrest-dependent-of-the-cdc25a-cdk2-cyclin-e-pathway
https://www.thailandmedical.news/news/breaking-covid-19-news-sars-cov-2-causes-macular-degeneration-by-promoting-rpe-cell-senescence-via-the-ros-p53-p21-pathway
