Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 19, 2024 1 year, 4 months, 1 week, 6 hours, 25 minutes ago
Mpox News: A team of researchers from Nazarbayev University in Astana, Kazakhstan, has made significant progress in understanding the monkeypox virus (MPXV) by exploring the roles of various proteins involved in its infection process. This comprehensive study delves into the intricate details of MPXV proteins and highlights the advancements in detection techniques that target these proteins. This
Mpox News report unpacks the study's findings, focusing on the key proteins that drive the virus's replication, spread, and interaction with host cells, while also discussing the latest detection methods that could prove critical in managing future outbreaks.
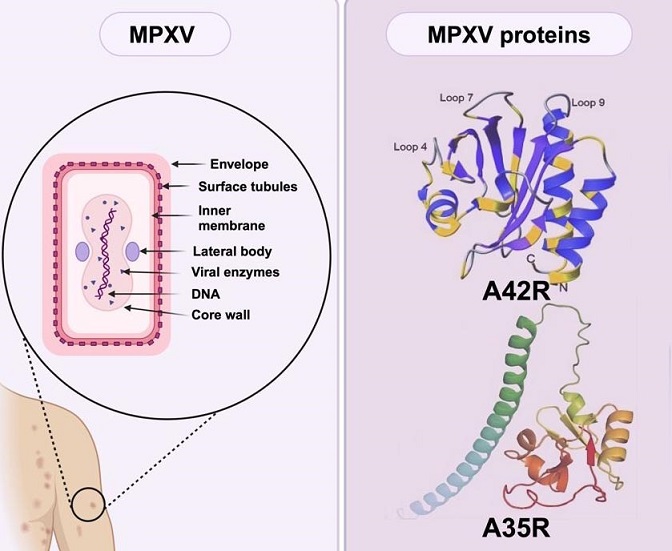 Graphical Abstract - Deep dive into Mpox (Monkey) virus proteins
The Importance of MPXV Proteins in Viral Replication and Spread
Graphical Abstract - Deep dive into Mpox (Monkey) virus proteins
The Importance of MPXV Proteins in Viral Replication and Spread
Monkeypox, caused by the MPXV, is a zoonotic disease that shares many clinical features with smallpox, making it difficult to diagnose based solely on symptoms. Understanding the proteins encoded by the MPXV genome is crucial, as these proteins are not only integral to the virus's ability to infect and spread within hosts but also serve as potential targets for diagnostic and therapeutic interventions.
The MPXV genome is a double-stranded DNA structure approximately 197 kilobases in length, encoding nearly 190 proteins. These proteins can be categorized based on their involvement in either the intracellular mature virus (IMV) form or the extracellular enveloped virus (EEV) form of the virus. Each protein plays a specific role in the virus's lifecycle, from attachment and entry into host cells to replication and eventual release.
IMV Proteins: The Pillars of Viral Stability and Replication
A29L: The Viral Attachment Specialist
The A29L protein, an ortholog of the vaccinia virus (VACV) A27 protein, is a crucial component of the IMV form of MPXV. It is primarily responsible for mediating the virus's attachment to host cells, a critical first step in the infection process. The A29L protein binds to glycosaminoglycans (GAGs) on the surface of host cells, facilitating the virus's entry. This interaction is vital for the virus's ability to infect and spread within the host.
Structurally, A29L consists of 110 amino acids, with a high degree of similarity (94.54%) to its VACV counterpart. The protein features an N-terminal signal peptide, a heparin-binding site (HBS), an alpha-helical coiled-coil domain, and a C-terminal anchoring domain. Despite a minor difference in the HBS sequence between A29L and VACV A27, both proteins exhibit similar binding affinities to heparin, a key component of the GAGs on host cell surfaces. This similarity underscores the potential for cross-reactive diagnostic tools and vaccines that target A29L.
H3L: Enhancing Viral Infectivity
The H3L protein, expressed in the IMV form, plays a pivotal role in enhancing the virus's infectivity by facilitating its binding to host cells. H3L
is not only a target for neutralizing antibodies but also triggers robust cellular immune responses, making it a key focus in vaccine development.
H3L contains 324 amino acids and shares 93.52% homology with its VACV counterpart. It includes at least two recognized human leukocyte antigen (HLA) class I-restricted T-cell epitopes, which are crucial for eliciting an immune response. Studies have shown that antibodies targeting H3L can protect against lethal MPXV challenges, highlighting its potential as a vaccine candidate.
Moreover, recent research has identified specific mutations in the H3L protein that may affect its recognition by the immune system. For instance, a mutation at the 233rd residue from alanine to serine was found to reduce the cross-reactivity of H3L with anti-serum elicited by the VACV TianTan strain. This finding is significant for developing more effective vaccines that provide broader protection against MPXV.
E8L: Facilitating Viral Entry
E8L is another critical IMV surface membrane protein, comprising 304 amino acids. It plays a significant role in the virus's ability to bind to host cells, specifically interacting with chondroitin, a type of GAG. This interaction is crucial for the virus's entry into host cells, making E8L a key target for detection and therapeutic strategies.
The structure of E8L includes three domains: the virion surface domain, the transmembrane domain, and the intra-virion domain. These structural features are essential for E8L's function in viral entry and its potential as a target for diagnostic tools. Recent studies have focused on the ganglioside-binding domain of E8L, identifying specific epitopes that could be used in vaccine formulations.
M1R: A Conserved Player in Viral Assembly
M1R, a myristoylated surface membrane protein, is highly conserved across Orthopoxviruses, sharing 98.4% homology with the VACV L1R protein. It is integral to the assembly and entry of viral particles, making it a crucial component of the IMV form.
Research has shown that M1R is a primary target for neutralizing antibodies, particularly in smallpox and cowpox viruses. This protein's role in virion assembly and its presence on the IMV surface make it a potent target for diagnostic and therapeutic interventions. Moreover, studies have demonstrated that M1R can be effectively used in vaccines, eliciting strong neutralizing antibody responses.
L1R: A Key Target for Neutralizing Antibodies
L1R is another essential IMV protein that plays a critical role in the virus's entry into host cells and the assembly of viral particles. This protein is highly conserved, making it a prime target for cross-reactive antibodies and vaccines.
L1R's structure includes a cluster of alpha-helices that wrap around beta-sheets, contributing to its stability and function. Despite its low affinity for GAGs compared to other MPXV proteins, L1R is crucial for the virus's ability to infect and spread within host organisms. Vaccines targeting L1R have shown promise in inducing long-lasting immune responses, making it a valuable component of future MPXV vaccines.
EEV Proteins: Facilitating Viral Spread and Immune Evasion
F13 (C19L): The Viral Packaging and Release Expert
The F13 protein, encoded by the MPXV C19L gene, is a vital component of the EEV form. This 372-amino-acid protein is located on the inner surface of the EEV's outer membrane and is responsible for viral packaging and release from host cells. F13 interacts with host membrane proteins such as Rab9 and TIP47 to form a virus-specific wrapping complex essential for the virus's spread.
F13 is also involved in lipid metabolism, further highlighting its multifaceted role in the virus's lifecycle. The protein's high degree of conservation between MPXV and VACV (99.9% similarity) makes it a promising target for antiviral drugs. Tecovirimat, an FDA-approved drug for smallpox, has shown potential in treating monkeypox by targeting the F13 protein.
A35R: Enhancing Cell-to-Cell Spread
A35R, an envelope glycoprotein, plays a crucial role in the formation of actin-containing microvilli, which facilitate the virus's spread from cell to cell. This protein shares 95.03% similarity with the VACV A33R protein and is involved in the effective transmission of viral particles across host tissues.
Recent studies have shown that A35R's localization to the plasma membrane is vital for its function in viral spread. The development of nanobodies targeting A35R has demonstrated high affinity and specificity, providing a basis for advanced diagnostic tools and potential therapeutic strategies.
B6R: Regulating Viral Spread and Immune Response
B6R is a glycoprotein that undergoes palmitoylation, a lipid modification essential for its localization on the EEV surface. This protein, homologous to the VACV B5R protein, plays a key role in the regulation of the host's complement system and the efficient spreading of the virus.
B6R is also involved in the wrapping of IMV to form EEV, a critical step in the virus's lifecycle. Detection methods targeting B6R have shown high specificity and sensitivity, making it a valuable marker for rapid MPXV diagnostics.
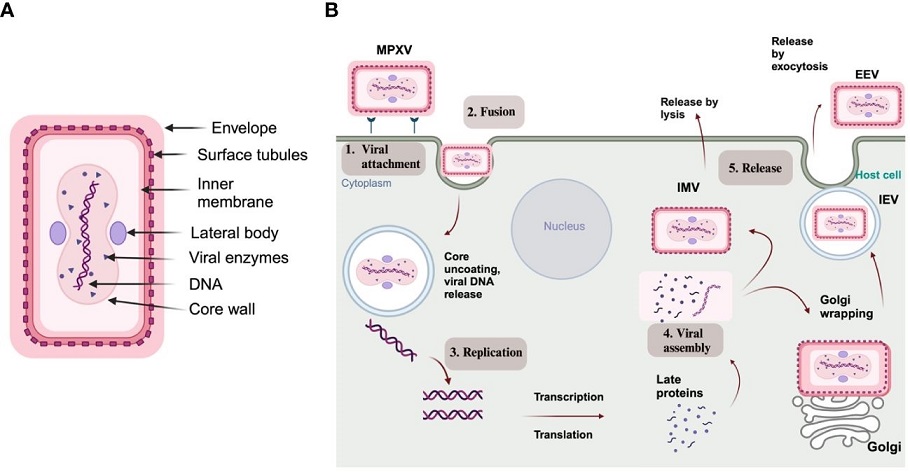 (A) Structure of MPXV; (B) Schematic representation of an MPXV replication cycle, including (1) viral attachment, (2) fusion, (3) replication, (4) viral assembly, and (5) release from the host cell
Profilin-like Protein A42R: A Unique and Lesser-Understood Target
(A) Structure of MPXV; (B) Schematic representation of an MPXV replication cycle, including (1) viral attachment, (2) fusion, (3) replication, (4) viral assembly, and (5) release from the host cell
Profilin-like Protein A42R: A Unique and Lesser-Understood Target
A42R, a profilin-like protein, stands out among MPXV proteins due to its unique structure and function. Unlike typical profilins, A42R exhibits weak affinity for actin and does not bind to poly (l-proline), which are key characteristics of other profilins. This protein is encoded by the gp153 locus of the MPXV genome and shares 98% similarity with VACV A42R.
The structure of A42R has been determined using X-ray diffraction, revealing a seven-stranded antiparallel beta-sheet surrounded by alpha helices. Despite its weak affinity for actin, A42R may interact with phosphatidylinositol lipids, suggesting a specialized role in the virus's lifecycle that is distinct from other viral proteins.
Understanding A42R's function is still in its early stages, but its unique characteristics make it a promising target for future research and potential therapeutic interventions.
Advances in Detection Techniques Targeting MPXV Proteins
The detailed characterization of MPXV proteins has led to the development of several advanced detection techniques. These methods are crucial for early diagnosis and controlling the spread of monkeypox, especially in regions where the virus has recently emerged.
Lateral Flow Assays (LFA): Rapid and Accessible Diagnostics
Lateral Flow Assays (LFA) have emerged as a key tool in the rapid detection of MPXV proteins, particularly A29L. These assays utilize colloidal gold nanoparticles to produce results within 10-15 minutes, offering a quick and cost-effective method for identifying MPXV infections.
LFAs targeting A29L have shown high specificity and sensitivity, making them a valuable tool for early diagnosis, especially in resource-limited settings. The double-antibody sandwich approach used in these assays ensures accuracy and reliability, crucial for managing outbreaks effectively.
Surface Plasmon Resonance (SPR): Detailed Protein Interactions
Surface Plasmon Resonance (SPR) is an advanced technique that allows for real-time, label-free analysis of molecular interactions. SPR has been used to study the binding affinities of MPXV proteins, such as A29L and L1R, with GAGs and other substrates.
This technique provides valuable insights into the protein interactions that drive MPXV's infection process, enabling the development of targeted diagnostic tools and therapeutic strategies. SPR's ability to quantify these interactions in real-time makes it a powerful tool for advancing our understanding of MPXV.
Surface-Enhanced Raman Spectroscopy (SERS): Precision in Protein Detection
Surface-Enhanced Raman Spectroscopy (SERS) is a highly sensitive technique that enhances the Raman signals of MPXV proteins, allowing for their detection at extremely low concentrations. SERS has been particularly effective in identifying multiple MPXV proteins, including A29L, M1R, B6R, and A35R, in serum samples. This technique's ability to detect MPXV proteins within minutes makes it a valuable tool for rapid diagnostics, especially in the early stages of infection when viral loads are low.
Electrochemical Biosensors: Portable and Efficient Diagnostics
Electrochemical biosensors offer a portable and efficient method for detecting MPXV proteins, making them ideal for point-of-care diagnostics. These sensors require minimal sample volumes and deliver results in under 15 minutes, making them highly suitable for rapid, on-site testing.
The development of a paper-based laser-scribed graphene (LSG) nanobiosensor for detecting the A29 protein is a notable advancement in this field. This sensor achieves detection limits as low as 3.0 × 10^−16 g/mL, demonstrating its potential for highly sensitive and specific MPXV diagnostics.
Conclusion and Future Directions
The detailed exploration of MPXV proteins by Nazarbayev University researchers has provided critical insights into the virus's mechanisms of infection and spread. By characterizing key proteins such as A29L, H3L, E8L, M1R, L1R, F13, A35R, B6R, and A42R, this study lays the groundwork for developing targeted diagnostic tools and therapeutic strategies.
While significant progress has been made, there is still much to learn about the functions of many MPXV proteins, particularly those with unique characteristics like A42R. Further research is essential to fully understand these proteins and their roles in the virus's lifecycle, which will be crucial for developing more effective diagnostics and treatments.
The study findings were published in the peer-reviewed journal: Frontiers in Cellular and Infection Microbiology.
https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2024.1414224/full
For the latest
Mpox News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/computational-study-identities-fda-approved-drugs-that-could-be-repurposed-to-treat-mpox-infections
https://www.thailandmedical.news/news/the-structural-and-functional-insights-of-the-e5-helicase-protein-of-the-mpox-virus
https://www.thailandmedical.news/news/the-mpox-virus-s-f3-protein-triggers-immune-responses-and-apoptosis
https://www.thailandmedical.news/news/unveiling-the-role-of-monkeypox-mpox-virus-a23-protein-in-human-cells

