Deficient Generation Of Spike-Specific Long-Lived Plasma Cells In The Bone Marrow After COVID-19
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 18, 2024 1 year, 2 months, 3 hours, 54 minutes ago
COVID-19 News: The COVID-19 pandemic has spurred intense research efforts to comprehend the intricacies of the immune response elicited by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Antibodies play a crucial role in protecting against infectious diseases, but the dynamics of their persistence vary widely among different pathogens. While antibodies against some viruses, such as measles and mumps, endure for a lifetime, those targeting influenza and COVID-19 tend to wane rapidly, with half-lives of less than a year. Unraveling the mystery behind the durability of antibody responses is imperative for understanding the long-term protection conferred by natural infection or vaccination. This
COVID-19 News report covers a study by researchers from the University of Maryland School of Medicine, Baltimore-USA who found that there is a deficient generation of spike-specific long-lived plasma cells in the bone marrow after COVID-19.
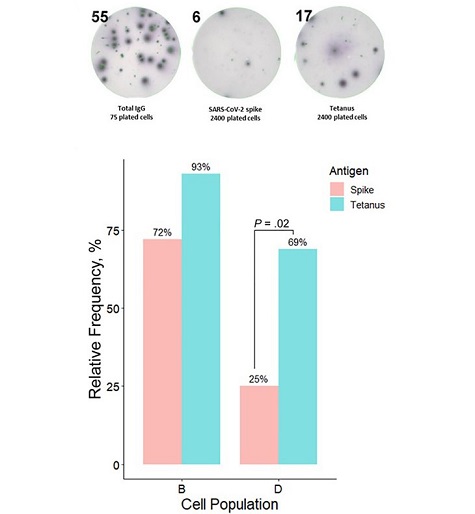 Bone marrow cellular subsets and their contributions to circulating antibody levels. A, Bone marrow anti-spike– and anti-tetanus–secreting plasma cells were assessed using enzyme-linked immunospot (ELISPOT) assays. A representative example shows ELISPOT findings for subset B in one of the studied individuals. Flow-sorted plasma cells were plated on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike trimer or tetanus toxoid plates; qualitative (based on presence or absence of antigen-specific plasma cells) and quantitative (based on antigen-specific immunoglobulin [Ig] G–secreting cells: total IgG–secreting cells ratio)
The Role of Long-Lived Plasma Cells
Bone marrow cellular subsets and their contributions to circulating antibody levels. A, Bone marrow anti-spike– and anti-tetanus–secreting plasma cells were assessed using enzyme-linked immunospot (ELISPOT) assays. A representative example shows ELISPOT findings for subset B in one of the studied individuals. Flow-sorted plasma cells were plated on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike trimer or tetanus toxoid plates; qualitative (based on presence or absence of antigen-specific plasma cells) and quantitative (based on antigen-specific immunoglobulin [Ig] G–secreting cells: total IgG–secreting cells ratio)
The Role of Long-Lived Plasma Cells
Stable, long-lived plasma cells (LLPCs) residing in the bone marrow are recognized as the linchpin of enduring antibody responses after infection or vaccination. These cells possess distinct morphological and transcriptomic signatures, surviving for decades and constituting the primary source of antibody-based serological memory. This study delves into the enigma of deficient generation of spike-specific LLPCs in the bone marrow following SARS-CoV-2 infection, shedding light on potential mechanisms contributing to waning antibody responses against the virus.
Research Methodology and Results
The research, conducted at the University of Maryland School of Medicine, focused on 20 individuals with a history of COVID-19 infection, examining bone marrow aspiration and plasma samples to characterize the antibody response. Notably, deficient generation of spike-specific LLPCs was observed in the bone marrow after severe infection, presenting a possible explanation for the decline in antibody responses to the spike protein after COVID-19.
The study utilized enzyme-linked immunospot assays to compare LLPC contributions to anti-tetanus and anti-spike antibodies. Intriguingly, while the model explained 98% of the observed variance in anti-tetanus immunoglobulin G (IgG) levels based on LLPC assays, the same model could not be fitted for anti-spike antibodies. This
disparity underscores the distinct nature of the immune response to SARS-CoV-2 and prompts further investigation into the underlying mechanisms.
Insights into Antibody Persistence
The research unveiled that, one-year post-infection, less than 1% of antibodies originated from LLPCs, with short-lived B cells being the primary source. This contrasts with tetanus-vaccinated patients, where LLPCs dominated antibody production one year after immunization, indicating a stark difference in the longevity of immune responses.
Discussion on Spike-Specific LLPC Deficiency
In contrast to tetanus toxoid, which induces a durable antibody response, SARS-CoV-2 fails to trigger the formation of a spike-specific LLPC compartment in the bone marrow. The deficiency in spike-specific LLPCs could account for the waning antibody responses to the spike protein observed after COVID-19. Importantly, individuals with a history of severe COVID-19 displayed an absence of the LLPC subpopulation in subset D, suggesting a potential predominance of extrafollicular immune responses in these patients, hindering the development of durable immune memory.
The study emphasizes the correlation between the tetanus-specific plasma subset D percentage and corresponding IgG levels, highlighting the crucial role of LLPCs in sustaining circulating antibodies long after exposure to tetanus antigen. However, this correlation was notably absent for COVID-19 antibodies, indicating a predominant contribution from short-lived plasma cells outside the bone marrow.
Mathematical Modeling and Immune Regulation
The study introduces a mathematical model proposing a testable mechanism of immune regulation. It suggests that competition for antigen between LLPCs and rapidly expanding short-lived B cells may inhibit LLPC expansion, resulting in a skewed B-cell population composition towards short-lived protection. This novel insight provides a potential explanation for the deficient generation of LLPCs in COVID-19.
Limitations and Future Directions
Acknowledging the study's limitations, including a small sample size and the absence of longitudinal sampling, the researchers advocate for further investigations. Future studies should explore potential causes for the decreased formation of LLPCs, trace the evolution of this cell population in the bone marrow over different time points before and after infection, and explore interventions to enhance LLPC formation following SARS-CoV-2 vaccination and other infectious diseases. Identifying blood markers associated with subset D in the bone marrow for noninvasive assessment of durable humoral immune responses is also emphasized.
Conclusion
In conclusion, the deficient generation of spike-specific LLPCs in the bone marrow after COVID-19 offers critical insights into the waning antibody responses observed in individuals with a history of SARS-CoV-2 infection. The distinct immune response dynamics uncovered in this study pave the way for further research and potential interventions to enhance the longevity of antibody responses, ultimately contributing to the global effort to combat the ongoing pandemic.
The study findings were published in the peer reviewed journal: The Journal of Infectious Diseases.
https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiad603/7606721
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
