Diabetes News: Salk Institute Creates Immune-Evading Stem Cells To Treat Type 1 Diabetes
Source: Diabetes News Aug 20, 2020 5 years, 4 months, 2 days, 9 hours, 47 minutes ago
Diabetes News: Researchers from Salk Institute-California have made a major breakthrough in the pursuit of a safe and effective treatment for type 1 diabetes, an illness that impacts an estimated 1.7 million Americans with a cost of US$14.5 billion annually.
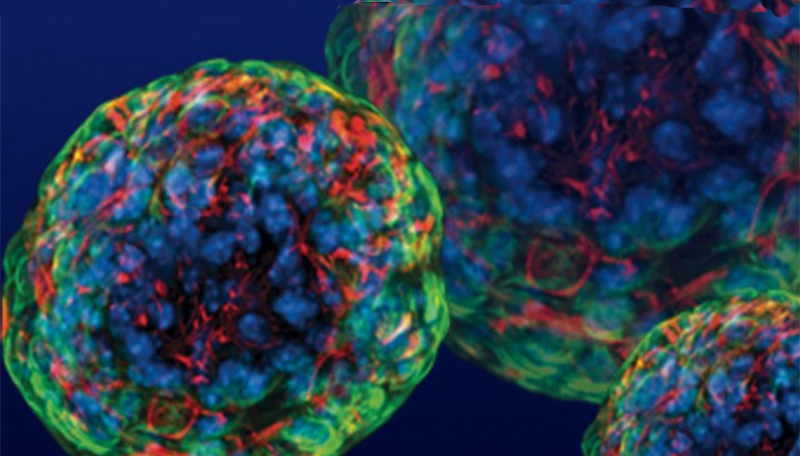
Utilizing stem cell technology, the research team generated the first human insulin-producing pancreatic cell clusters able to evade the immune system. These "immune shielded" cell clusters controlled blood glucose without immunosuppressive drugs once transplanted in the body.
The research findings were published in the journal: Nature
https://www.nature.com/articles/s41586-020-2631-z
Salk Professor Dr Ronald Evans, senior author and holder of the March of Dimes Chair in Molecular and Developmental Biology told Thailand Medical News, "Unfortunately most type 1 diabetics are children and teenagers. This is a disease that is historically hard to manage with drugs. We hope that regenerative medicine in combination with immune shielding can make a real difference in the field by replacing damaged cells with lab-generated human islet-like cell clusters that produce normal amounts of insulin on demand."
Unknown to many, type 1 diabetes is a lifelong condition that is challenging to manage, even with automated devices that deliver insulin to regulate blood sugar. Transplants of pancreatic beta islets ie clusters of cells that make insulin and other hormones from donor tissue can provide a cure, but require patients to take life-long immunosuppressing drugs, which carry serious risks.
For many years now, medical scientist have sought a better way to replenish lost pancreatic cells. Now, device-free transplantation of insulin-producing cells like these brings us a step closer to curing the disease, according to the lab.
In past studies, the Evans lab overcame an impediment in the field, in which stem-cell-derived beta-like cells produced insulin, but were not functional. The cells did not release insulin in response to glucose, as they were simply under powered, according to Evans.
The study team then discovered a genetic switch called ERR-gamma that when flipped, "turbo-charges" the cells.
Dr Michael Downes, a Salk senior staff scientist and co-author of both studies said,"When we add ERR-gamma, the cells have the energy they need to do their job. These cells are healthy and robust and can deliver insulin when they sense high glucose levels."
A major issue of the new research was to develop a way to grow beta-like cells in a three-dimensional environment that approximates the human pancreas. This gave the cells an islet-like property.
Significantly, the team discovered that a protein called WNT4 was able to turn on the ERR-gamma-driven maturation switch. This combination of steps generated functional cell clusters that mimic human islets: so-called human islet-like organoids (HILOs).
The study team next tackled the complex issue of immune rejection. Normal tissue transplants require lifelong immune suppressive the
rapies to protect the tissue from being attacked by the immune system; however these therapies also increase the risk for infections.
However inspired by the successes of immunotherapy drugs for cancer, the team initially showed that the checkpoint protein PD-L1 protected the transplanted cells.
First author Dr Eiji Yoshihara, a former staff scientist in the lab added, "By expressing PD-L1, which acts as an immune blocker, the transplanted organoids are able to hide from the immune system."
Dr Yoshihara then developed a method to induce PD-L1 in HILOs with short pulses of the protein interferon gamma. When transplanted into diabetic mice, these immune-evasive HILOs provided sustained blood glucose control in diabetic mice with healthy immune systems.
Dr Downes explains, "This is the first study to show that you can protect HILOs from the immune system without genetic manipulation. If we are able to develop this as a therapy, patients will not need to take immune-suppressing drugs."
However the team warned that more research needs to be done before this platform can be advanced to clinical trials.
First the transplanted organoids need to be tested in mice for longer periods of time to confirm that their effects are long-lasting.
Also additional work needs to be done to ensure they would be safe to use in humans as well.
Dr Evans concluded, "Fortunately we now have a product that could potentially be used in patients without requiring any kind of device ad it will improve the quality of their lives a whole lot."
For the latest
Diabetes News, keep on logging to Thailand Medical News.
