Drug Recall Alert: Contamination Prompts Recall Of Robitussin Honey CF Max Day Adult Cough Syrup By Haleon
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 27, 2024 2 years, 1 month, 1 week, 2 days, 21 hours, 37 minutes ago
Drug Recall Alert: In a recent development, UK-based healthcare company Haleon has issued a voluntary nationwide recall in the United States for certain batches of Robitussin cough syrups. The recall comes in response to the detection of microbial contamination in eight batches of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult. The affected products have expiration dates ranging from October 2025 to June 2026. This
Drug Recall Alert report delves into the details surrounding the recall, potential risks, and the company's response to safeguard public health.
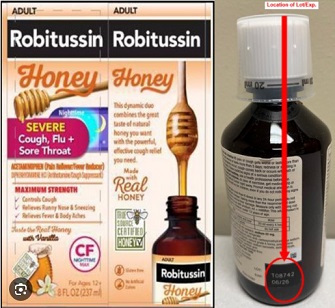 Contamination Prompts Recall Of Robitussin Adult Cough Syrup By Haleon
Contamination Prompts Recall Of Robitussin Adult Cough Syrup By Haleon
As rising COVID-19 and various other respiratory infections are causing many to develop coughs leading to brisk sales of cough syrups, amidst this recall, consumers need to pay attention and make sure that they do not consume the product mentioned.
Details of the Recall
Haleon, the manufacturer of Robitussin cough syrups, initiated the recall after identifying microbial contamination in specific lots of their products. The recall specifically targets Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult, both available in 4-ounce and 8-ounce doses. The company emphasizes that the voluntary recall is limited to batches manufactured and sold in the United States.
Potential Risks and Health Concerns
According to the company's statement, individuals with compromised immune systems face an elevated risk of severe or life-threatening adverse events due to microbial contamination. Conditions such as fungemia, characterized by the presence of fungi or yeasts in the blood, may arise in immunocompromised individuals who use the affected products. Symptoms of fungemia include chronic fatigue, severe confusion, non-healing wounds, irregular discharge, sweating, and itching.
Furthermore, the use of contaminated cough syrups by immunocompromised individuals could potentially lead to disseminated fungal infections. This serious condition occurs when fungi spread throughout the body, causing skin ulcers, abscesses, fever, bone lesions, or even meningitis. While the risk of life-threatening infections is lower for non-immunocompromised individuals, Haleon acknowledges that the occurrence of infections requiring medical intervention cannot be completely ruled out.
Company's Response and FDA Involvement
Haleon is taking proactive measures to address the recall, notifying distributors and customers directly. The company has provided clear instructions for the return of all recalled products. Consumers who have purchased the affected cough syrups are urged to cease consumption immediately.
The recall is being conducted with the knowledge and cooperation of the U.S. Food and Drug Administration (FDA). Haleon assures the public that, as of now, there have been no reports of adverse events related to the recalled products. The company's commitment t
o consumer safety is evident in its swift action to mitigate potential health risks.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/haleon-issues-voluntary-nationwide-recall-robitussin-honey-cf-max-day-adult-and-robitussin-honey-cf
Comparison with Recent Drug Recalls
The recall of Robitussin cough syrups adds to the growing list of drug recalls in the United States. Since January, there have been at least four recalls, encompassing products such as lubricant eye drops, various intravenous (IV) bags, and Zenzedi or dextroamphetamine sulfate tablets. Reasons for these recalls varied, including concerns about device and drug safety, the potential for super-potent drugs, and issues with mislabeled packaging.
In 2023, most of the drug recalls in the United States were drugs and medications from India due to product contamination.
Among the most media covered recall was contaminated eye drops made in India that led to many Americans being blinded and individuals even dying!
https://www.thailandmedical.news/news/breaking-u-s-medical-news:-three-deaths,-eight-cases-of-blindness,-hundreds-of-americans-infected-due-to-contaminated-eyedrops-from-india
Conclusion
The recall of Robitussin Honey CF Max Day Adult and Robitussin Honey CF Max Nighttime Adult by Haleon highlights the importance of vigilant monitoring and swift action in the pharmaceutical industry. With a focus on consumer safety, the company's voluntary recall underscores the potential health risks associated with microbial contamination in cough syrups. Individuals who have purchased the affected products are strongly advised to follow the company's instructions for return and contact the consumer relations team for further assistance. As the situation evolves, ongoing cooperation between healthcare companies and regulatory authorities remains crucial to ensuring public health and safety.
For the latest
Drug Recall Alerts, keep on logging to Thailand Medical News.
