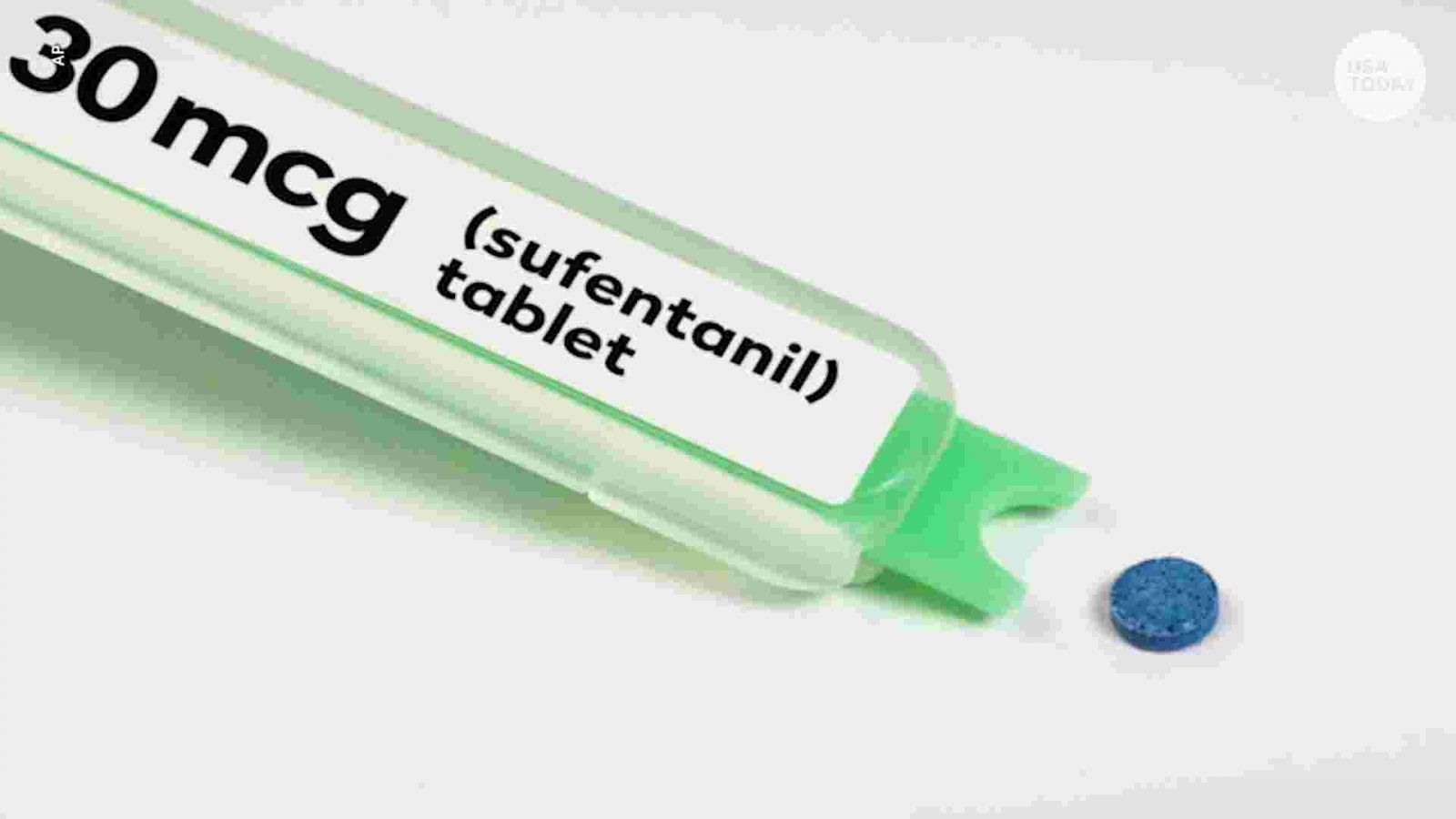
Dsuvia which is 10 times stronger than Fentanyl and 1000 times stronger than Morphine, approved by US FDA and EU.
Thailand Medical News Jan 19, 2019 6 years, 3 months, 1 week, 11 hours, 27 minutes ago
The US FDA and EU has approved a new sublingual formulation of sufentanil, Dsuvia, for the management of acute pain in adults in medically supervised healthcare settings, such as hospitals, surgical centers, and emergency departments. The drug is supplied in a 30 microgram tablet in a single-dose, prefilled applicator for administration by a healthcare professional, and it will not be available in retail
pharmacies or for outpatient use.
The drug was developed in collaboration with the Department of Defense with the military population in mind. AcelRx Pharmaceticals, maker of the drug, explains that, in many care settings—including battlefield settings—patients may not have readily available access to intravenous (IV) treatments for pain, and intramuscular injections (currently the standard of care for battlefield patients) are not as effective as IV options at providing timely relief, and may not be effective in cases of severe trauma that involves hypovolemic shock.
As such, "We believe the unique features of Dsuvia are an important leap forward in the management of acute pain and patient care in these settings,” said AcelRx’s CEO, Vince Angotti, in a statement. Angotti noted that the drug will be subject to a Risk Evaluation and Mitigation Strategy program. As part of the program, the drug maker will monitor distribution and audit wholesalers’ data, evaluate proper use in the healthcare setting, and monitor for diversion or abuse and decertify any healthcare setting that is noncompliant.
The approval of Dsuvia was based on the results of 3 phase 3 clinical trials. The first, SAP 301, was conducted in patients with moderate to severe acute pain after abdominal surgery. The study demonstrated that patients receiving the drug experienced significantly greater pain reduction versus placebo over the first 12 hours post-treatment. In SAP 302, conducted in adult patients in the emergency department who had moderate to severe acute pain associated with trauma or injury, the drug was demonstrated to reduce pain, and a cognitive assessment found that treatment was not associated with drug-induced cognitive impairment. Finally, the open-label SAP303 study in patients with moderate to severe acute pain after surgery found that safety and efficacy of the drug were similar among patients regardless of their age, renal function, or liver function.
Approval of the drug was highly criticized amidst an opioid crisis in the US. Raeford Brown, MD, chair of the FDA’s Anesthetic and Analgesic Drug Products Committee, urged the FDA not to approve Dsuvia. Writing to the FDA, Brown called the product “an extremely divertible drug,” and predicted that diversion, abuse, and death would be observed within the first months of the drug’s presence on the market.
In a statement, FDA Commissioner Scott Gottlieb, MD, sought to deflect criticism of the approval, saying that, “Looking beyond this particular drug approval, I believe that we should consider whether we should be doing more to evaluate each candidate opioid, not just as an independent review decision, but rather also to consider each novel opioid drug in the context of the overall therapeutic armamentarium that’s available to patients and providers.”
According
to Gottlieb, “This opioid formulation, along with Dsuvia’s unique delivery device, was a priority medical product for the Pentagon because it fills a specific and important, but limited, unmet medical need in treating our nation’s soldiers on the battlefield.”
Side effects of the potent drug includes extreme tiredness, breathing problems, coma and death.