Dysfunctions in the Vagus Nerve, Hypothalamic-Pituitary-Adrenal Axis and Mitochondria Underlie the Complexities of Long COVID
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 14, 2024 1 year, 3 weeks, 14 hours, 30 minutes ago
Medical News: Long COVID is a complex and often debilitating condition that continues to affect millions of people worldwide. Defined by lingering symptoms lasting for months - or even years - after recovery from the initial SARS-CoV-2 infection, it has become a global health concern. The symptoms of long COVID range from fatigue and brain fog to respiratory issues and cardiac dysfunction, affecting individuals' ability to work, care for their families, and lead normal lives. Despite extensive research, the underlying causes of long COVID have remained elusive.
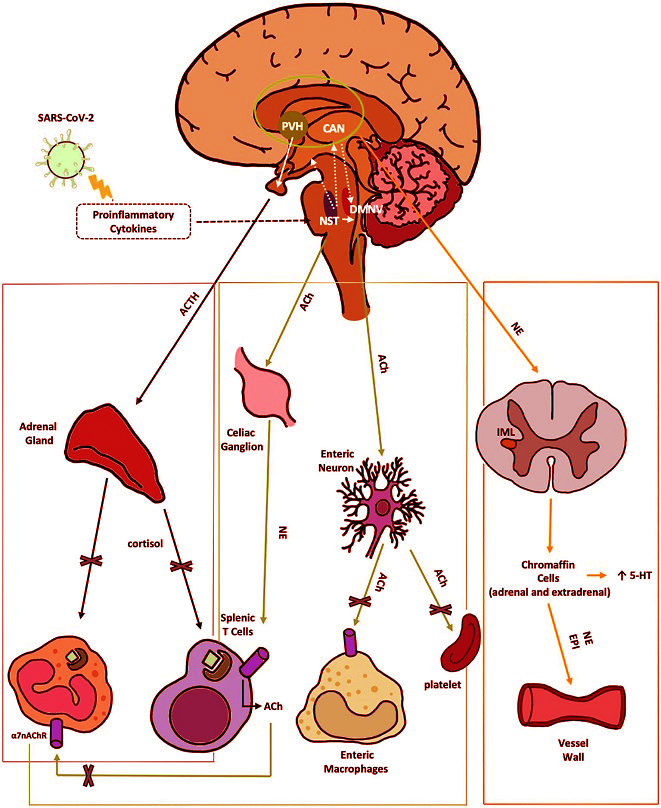 Anti-inflammatory pathways of the VN disrupted by SARS-CoV-2. 1) The HPA axis reflex (black) depicts the suppression of the hypothalamic-pituitary-adrenal axis reflex, leading to a reduction in cortisol production by SARS-CoV-2. This reduction in cortisol impairs the regulation of both innate and adaptive immune responses. Nerve fibers from the NTS stimulate the release of CRH by neurons in the PVH, a hypothalamic nucleus within the central autonomic network. The CAN, comprised of the thalamus, amygdala, hypothalamus, and brainstem nuclei, integrates emotional, sensory, and cognitive stimuli to produce autonomic and endocrine responses. 2) The cholinergic anti-inflammatory reflex (green) illustrates the VN projection to the gastrointestinal tract. This reflex is initiated by afferent fibers from the NTS, which relay peripheral visceral sensory information to the DMNV. ACh, released from DMNV efferent fibers, finally inhibits cytokine release from intestinal macrophages, thereby mitigating local inflammation. Moreover ACh suppress platelets activation. 3) The vago-sympathetic pathway (orange) is activated by vagal efferent stimuli originating from the NTS. This pathway regulates sympathetic outflow, thought the CAN. It targets preganglionic sympathetic neurons in the IML of the spinal cord and chromaffin cells. This complex network modulates peripheral blood tone, vasoconstriction, and immune responses. Enterochromaffin cells are involved in the gut metabolism of 5-HT. α7-nAChR, αlpha7-nicotinicACh-Receptor; ACh, acetylcholine; ACTH, adrenocorticotropic hormone; CAN, central autonomic network; DMNV, dorsal motor nucleus of vagus nerve; EPI, epinephrine; HPA, hypothalamic–pituitary–adrenal; IML, intermediolateral nucleus; NE, norepinephrine; NTS, nucleus tractus solitarius; PVH, parvo-cellular nucleus; VN, vagus nerve; 5-HT, serotonin.
Anti-inflammatory pathways of the VN disrupted by SARS-CoV-2. 1) The HPA axis reflex (black) depicts the suppression of the hypothalamic-pituitary-adrenal axis reflex, leading to a reduction in cortisol production by SARS-CoV-2. This reduction in cortisol impairs the regulation of both innate and adaptive immune responses. Nerve fibers from the NTS stimulate the release of CRH by neurons in the PVH, a hypothalamic nucleus within the central autonomic network. The CAN, comprised of the thalamus, amygdala, hypothalamus, and brainstem nuclei, integrates emotional, sensory, and cognitive stimuli to produce autonomic and endocrine responses. 2) The cholinergic anti-inflammatory reflex (green) illustrates the VN projection to the gastrointestinal tract. This reflex is initiated by afferent fibers from the NTS, which relay peripheral visceral sensory information to the DMNV. ACh, released from DMNV efferent fibers, finally inhibits cytokine release from intestinal macrophages, thereby mitigating local inflammation. Moreover ACh suppress platelets activation. 3) The vago-sympathetic pathway (orange) is activated by vagal efferent stimuli originating from the NTS. This pathway regulates sympathetic outflow, thought the CAN. It targets preganglionic sympathetic neurons in the IML of the spinal cord and chromaffin cells. This complex network modulates peripheral blood tone, vasoconstriction, and immune responses. Enterochromaffin cells are involved in the gut metabolism of 5-HT. α7-nAChR, αlpha7-nicotinicACh-Receptor; ACh, acetylcholine; ACTH, adrenocorticotropic hormone; CAN, central autonomic network; DMNV, dorsal motor nucleus of vagus nerve; EPI, epinephrine; HPA, hypothalamic–pituitary–adrenal; IML, intermediolateral nucleus; NE, norepinephrine; NTS, nucleus tractus solitarius; PVH, parvo-cellular nucleus; VN, vagus nerve; 5-HT, serotonin.
A groundbreaking study led by researchers from the National Institute for Infectious Diseases Lazzaro Spallanzani in Rome, Italy, sheds new light on this perplexing condition. The team proposes a hypothesis connecting long COVID pathogenesis to the dysfunction of three critical systems: the vagus nerve, the hypothalamic-pituitary-adrenal (HPA) axis, and mitochondria. These systems form an interconnected neuro-endocrine-metabolic axis, which, when disrupted, can lead to the persistent symptoms associated with long COVID. This
Medical News report explores their findings and their implications in detail.
The Vagus Nerve: The Body's Communication Highway
The vagus nerve plays a crucial role in regulating the body
’s anti-inflammatory responses and maintaining homeostasis. Connecting the brain to vital organs such as the heart, lungs, and gastrointestinal tract, the vagus nerve acts as a communication superhighway, transmitting signals that help control heart rate, digestion, and immune responses.
In individuals with long COVID, the vagus nerve appears to be a primary target of SARS-CoV-2. The researchers identified two key mechanisms through which the virus damages this nerve: direct viral invasion and immune-mediated damage.
Post-mortem analyses of COVID-19 patients revealed significant neuroinflammation in vagus nerve tissue, with viral particles present within the nerve itself. In addition, autoantibodies targeting receptors on the vagus nerve were identified, impairing its ability to regulate immune responses.
This damage has far-reaching consequences. Vagus nerve dysfunction reduces the body’s ability to suppress inflammation, leading to a state of chronic systemic inflammation. This persistent inflammatory state is thought to be a major driver of long COVID symptoms such as fatigue, muscle pain, and cognitive dysfunction. Moreover, reduced vagal tone - a measure of vagus nerve activity - has been linked to impaired mitochondrial function and hormonal imbalances, creating a cascade of dysfunctions.
The Hypothalamic-Pituitary-Adrenal Axis: Stress Hormones Gone Awry
The hypothalamic-pituitary-adrenal (HPA) axis is another critical system impacted in long COVID. This axis regulates the production of cortisol, a hormone essential for managing stress and inflammation. In healthy individuals, the vagus nerve supports the HPA axis by triggering the release of corticotrophin-releasing hormone (CRH) from the hypothalamus. CRH stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH), which in turn prompts the adrenal glands to produce cortisol.
However, in long COVID patients, this delicate balance is disrupted. The study revealed that SARS-CoV-2 damages the adrenal glands, reducing their ability to produce cortisol. Autopsy findings have shown widespread fibrosis and structural damage in the adrenal glands of COVID-19 patients. Additionally, the virus may cause long-term epigenetic changes to the glucocorticoid receptor, reducing its sensitivity to cortisol. As a result, long COVID patients often exhibit abnormally low cortisol levels and a blunted stress response.
The consequences of HPA axis dysfunction are profound. Without sufficient cortisol, the body struggles to regulate inflammation, leading to the persistence of inflammatory markers such as cytokines. This hormonal imbalance exacerbates symptoms such as fatigue, muscle weakness, and mood disturbances, which are commonly reported in long COVID patients.
Mitochondria: The Energy Powerhouses Under Siege
Mitochondria, the organelles responsible for producing energy in cells, are another critical target of SARS-CoV-2. These tiny structures play a central role in cellular metabolism, immune regulation, and stress responses. During a viral infection, mitochondria typically activate the body’s antiviral defenses. However, SARS-CoV-2 appears to exploit and damage mitochondria for its replication.
The researchers identified several mechanisms through which SARS-CoV-2 disrupts mitochondrial function. The virus’s spike protein impairs genes involved in mitochondrial energy production, reducing the production of adenosine triphosphate (ATP), the cell’s primary energy currency. At the same time, the virus increases the production of mitochondrial reactive oxygen species (mtROS), which contribute to oxidative stress and cellular damage.
In long COVID patients, mitochondrial dysfunction persists long after the acute infection has resolved. This dysfunction leads to energy deficits and exacerbates systemic inflammation, creating a vicious cycle that perpetuates symptoms such as fatigue and brain fog. The researchers also noted that mitochondrial damage may disrupt the synthesis of acetylcholine, a neurotransmitter critical for vagus nerve function, further amplifying the cascade of dysfunctions.
The Interconnected Web of Dysfunction
The study highlights the intricate interplay between the vagus nerve, HPA axis, and mitochondria. These systems are deeply interconnected, and dysfunction in one system can cascade into the others. For example, vagus nerve dysfunction reduces cortisol production and impairs mitochondrial function. In turn, mitochondrial dysfunction exacerbates inflammation and disrupts the synthesis of acetylcholine, which is essential for vagus nerve signaling.
This interconnected web of dysfunction creates a self-perpetuating cycle that makes recovery from long COVID challenging. The researchers propose that this cycle may explain why long COVID symptoms persist for months or even years in some individuals.
Implications for Treatment
The findings of this study have significant implications for the treatment of long COVID. By identifying the vagus nerve-HPA-mitochondrial axis as a central mechanism in long COVID pathogenesis, the researchers have opened new avenues for therapeutic intervention. Potential treatments could include:
-Vagus Nerve Stimulation (VNS): Non-invasive VNS has shown promise in improving symptoms such as fatigue, brain fog, and mood disturbances in long COVID patients. By enhancing vagal tone, VNS may help restore balance to the neuro-endocrine-immune axis.
-Mitochondrial Support Therapies: Therapies aimed at restoring mitochondrial function, such as antioxidant supplementation or targeted metabolic interventions, could help alleviate fatigue and improve energy levels.
-Hormonal Interventions: Treatments that restore cortisol levels or enhance glucocorticoid receptor sensitivity may help regulate inflammation and improve overall recovery.
Conclusions
The study by the team at the National Institute for Infectious Diseases Lazzaro Spallanzani offers a comprehensive framework for understanding the pathogenesis of long COVID. By linking vagus nerve dysfunction, HPA axis impairment, and mitochondrial damage, the researchers provide a unified model that explains the diverse and persistent symptoms of this condition.
In conclusion, addressing the interconnected dysfunctions of the vagus nerve, HPA axis, and mitochondria could hold the key to effective long COVID treatments. This holistic approach recognizes the complex interplay between the body’s systems and underscores the importance of personalized, multidimensional therapeutic strategies. Moving forward, integrating these insights into clinical practice may offer hope to the millions of individuals grappling with long COVID.
The study findings were published in the peer-reviewed journal: Frontiers in Cellular and Infection Microbiology.
https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2024.1501949/full
For the latest on Long COVID, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/how-autoimmune-responses-in-the-brain-could-explain-long-covid-symptoms
https://www.thailandmedical.news/news/latest-american-study-validates-that-brain-changes-linked-to-long-covid-symptoms
https://www.thailandmedical.news/news/illinois-study-finds-that-covid-19-vaccines-do-not-prevent-long-covid-neurological-issues
https://www.thailandmedical.news/articles/long-covid
