Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 27, 2024 3 months, 3 weeks, 5 days, 9 hours, 59 minutes ago
Medical News: A recent study led by researchers from the Department of Natural Sciences at the University of Michigan-Dearborn-USA has provided significant insights into how the acidic environment of early endosomes impacts the journey of SARS-CoV-2 within host cells. The findings offer a potential pathway for developing treatments to impede the virus from successfully infecting human cells.
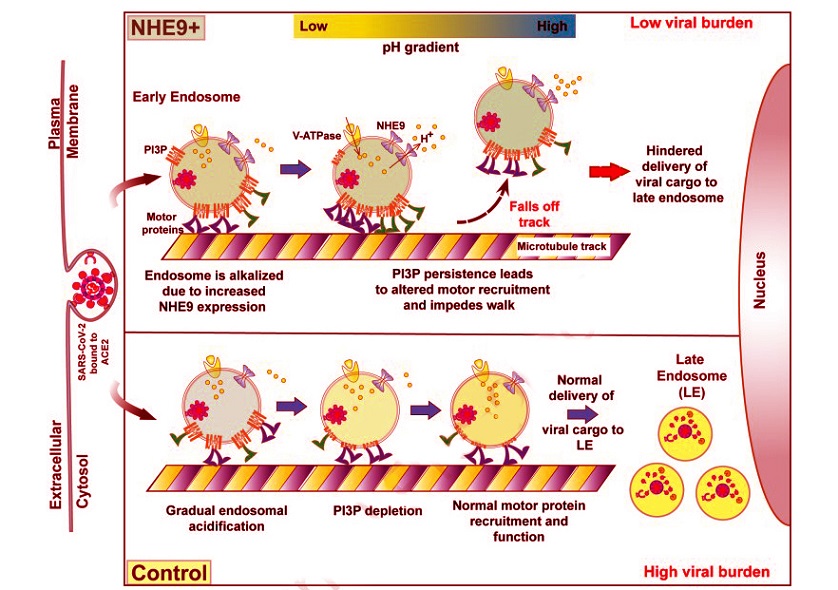 Schematic illustrating the impairment of SARS-CoV-2 entry into host cells due to NHE9-mediated luminal alkalization.Early endosomes (EEs) carrying the endocytosed SARS-CoV-2 virus bound to the ACE2 receptor are shown on microtubules. Endosomal acidification is regulated by V-ATPase, which pumps protons into the endosomal lumen, and NHE9 alkalizes the lumen by moving protons out of the endosome. Phosphatidylinositol-3-phosphate (PI3P) on early endosomes plays a crucial role in the recruitment of motor proteins important for the movement of early endosomes towards the late endosomes located at the perinuclear regions of the cell. Under normal conditions (Control, bottom panel), as the early endosome matures, its lumen acidifies and PI3P levels on the membrane are depleted over time. This decrease in PI3P levels is essential for the proper maturation and perinuclear trafficking of viral cargo to late endosomes. When the lumen of the early endosome is alkalized due to increased expression of NHE9 (NHE9+, top panel), it leads to increased retention of PI3P on early endosome membranes. This plausibly leads to altered recruitment disrupting the balance and coordination of the oppositely directed motor proteins, which rely on tightly regulated cycles of attachment and detachment from microtubules. These cycles are typically influenced by the lipid composition of endosomal membranes and associated regulatory proteins. Disruptions caused by persistent PI3P increase the frequency of detachment from microtubules, decreased run length and velocity of EE movement. This inefficient transport of viral cargo to late endosomes ultimately contributes to a decreased viral burden in host cells.
Schematic illustrating the impairment of SARS-CoV-2 entry into host cells due to NHE9-mediated luminal alkalization.Early endosomes (EEs) carrying the endocytosed SARS-CoV-2 virus bound to the ACE2 receptor are shown on microtubules. Endosomal acidification is regulated by V-ATPase, which pumps protons into the endosomal lumen, and NHE9 alkalizes the lumen by moving protons out of the endosome. Phosphatidylinositol-3-phosphate (PI3P) on early endosomes plays a crucial role in the recruitment of motor proteins important for the movement of early endosomes towards the late endosomes located at the perinuclear regions of the cell. Under normal conditions (Control, bottom panel), as the early endosome matures, its lumen acidifies and PI3P levels on the membrane are depleted over time. This decrease in PI3P levels is essential for the proper maturation and perinuclear trafficking of viral cargo to late endosomes. When the lumen of the early endosome is alkalized due to increased expression of NHE9 (NHE9+, top panel), it leads to increased retention of PI3P on early endosome membranes. This plausibly leads to altered recruitment disrupting the balance and coordination of the oppositely directed motor proteins, which rely on tightly regulated cycles of attachment and detachment from microtubules. These cycles are typically influenced by the lipid composition of endosomal membranes and associated regulatory proteins. Disruptions caused by persistent PI3P increase the frequency of detachment from microtubules, decreased run length and velocity of EE movement. This inefficient transport of viral cargo to late endosomes ultimately contributes to a decreased viral burden in host cells.
The research team, including Perla Fares, Mariam Duhaini, Suvranta K. Tripathy, Ali Srour, and Kalyan C. Kondapalli, explored the mechanisms that govern the intracellular transport of SARS-CoV-2 after it attaches to its host receptor, ACE2. This
Medical News report delves into their groundbreaking discoveries, emphasizing the therapeutic potential of targeting pH regulation within endosomes.
Understanding Viral Entry and Transport
SARS-CoV-2 uses a receptor-mediated endocytosis pathway to infiltrate human cells. This process begins with the virus binding to the ACE2 receptor, which triggers its encapsulation into early endosomes. As these endosomes progress toward late endosomes, their internal pH decreases, creating an acidic environment essential for viral genome release. This step enables the virus to replicate and produce new viral particles.
The research highlights the pivotal role of the sodium-proton exchanger NHE9, a protein responsible for modulating the pH within endosomes. By limiting the acidification of early endosomes, NHE9 hinders the ability of SARS-CoV-2 to reach its replication site. The study
’s findings open doors for innovative therapeutic strategies that could block the virus’s progression at an early stage.
The Role of NHE9 in Viral Inhibition
In their experiments, the researchers used HEK293T cells engineered to express ACE2 (HEK293T-hACE2) to investigate the impact of NHE9 on the virus’s journey through the endosomal pathway. They observed that overexpression of NHE9 increased the pH within early endosomes, disrupting the virus’s ability to transition to late endosomes. Fluorescent imaging techniques revealed that spike proteins from SARS-CoV-2 were less likely to colocalize with late endosomal markers in cells with elevated NHE9 levels.
The persistence of phosphatidylinositol-3-phosphate (PI3P) on the membranes of early endosomes was identified as a key factor influenced by NHE9. PI3P, a lipid crucial for recruiting motor proteins, plays an integral role in the movement of endosomes along microtubules. In cells with heightened NHE9 activity, PI3P levels remained elevated for longer periods, causing disruptions in endosomal transport.
Experimental Evidence
To further validate their findings, the researchers engineered cells to overexpress a mutant form of NHE9 incapable of modulating pH. These cells displayed normal pH levels within early endosomes, and the transport of spike proteins was unaffected. This confirmed that NHE9’s ability to regulate pH is central to its role in impairing viral trafficking.
Additional experiments were conducted using a pseudotyped virus that mimics SARS-CoV-2. The results demonstrated a significant reduction in viral infectivity in cells overexpressing NHE9. Specifically, the viral burden was reduced by over 70% compared to control cells, highlighting the protein’s potential as a therapeutic target.
Broader Implications
The study’s findings extend beyond SARS-CoV-2. Many viruses rely on endocytic pathways to enter host cells, and targeting pH regulation within endosomes could offer a universal strategy for combating a range of viral infections. The researchers propose that therapies designed to modulate NHE9 expression or mimic its effects could become effective tools in the fight against viral diseases.
Conclusion
The implications of this research are profound. By identifying the role of NHE9 in regulating endosomal pH and its subsequent impact on viral transport, the study provides a novel avenue for therapeutic intervention. The targeted manipulation of endosomal pH represents a promising strategy for mitigating the spread of SARS-CoV-2 and potentially other viruses that exploit similar mechanisms for entry.
The study concludes that enhancing NHE9 expression or developing drugs to mimic its pH-regulating effects could serve as a viable approach to limiting viral infections. Furthermore, the persistence of PI3P in early endosomes and its impact on motor protein recruitment underscores the complexity of intracellular viral transport and the potential for disrupting these processes at multiple levels.
The study findings were published in the peer-reviewed Journal of Biological Chemistry.
https://www.sciencedirect.com/science/article/pii/S0021925824026462
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-spike-protein-accelerates-aging-of-blood-vessels
https://www.thailandmedical.news/news/covid-19-news-impact-of-sars-cov-2-on-cardiac-health-czech-republic-study-reveals-alarming-findings-on-ventricular-and-atrial-strain
https://www.thailandmedical.news/news/covid-19-causes-inflammation-tissue-injury-and-fibrosis-of-the-respiratory-tract-and-lungs
https://www.thailandmedical.news/articles/coronavirus
