Nikhil Prasad Fact checked by:Thailand Medical News Team Apr 21, 2024 1 year, 9 months, 3 weeks, 4 days, 10 hours, 50 minutes ago
COVID-19 News: The aftermath of SARS-CoV-2 infection or COVID-19 vaccination often leads to a condition known as "long-haul syndrome." This syndrome, affecting about 10-35% of individuals who have been exposed to the SARS-CoV-2 virus, manifests as persistent fatigue, cognitive impairment, cardiac issues, and other systemic complications lasting for months or even years. Exploring the underlying mechanisms of long-COVID is crucial for effective management and prevention strategies.
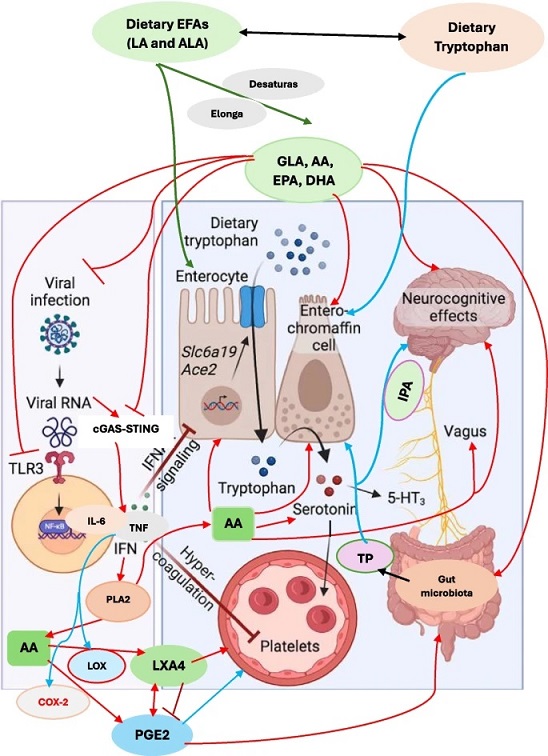 Scheme showing potential interaction between EFAs and their metabolites and serotonin. Indoles are synthesized from tryptophan by gut microbiota that express tryptophanase (TP). Indoles are cytoprotective molecules. Indolepropionic acid (IPA) synthesized by gut microbiota is a neuroprotective substance and binds to the pregnane X receptor (PXR) in intestinal cells, to facilitate mucosal homeostasis. IPA absorbed from the gut is transferred to the brain to prevent β-amyloid fibril formation. Tryptophan is metabolized to indole-3-aldehyde (I3A) by gut microbiota that acts on the aryl hydrocarbon receptor (AhR) in intestinal immune cells. Gut microbiota have the ability to alter the expression of serotonin-related genes and thus, alters its (serotonin) biosynthesis. Gut microbiota (i) directly act on enterochromaffin (EC) cells to increase colonic tryptophan hydroxylase 1 (Tph1) expression and promote serotonin synthesis; (ii) alter host by direct action or through their metabolites, short chain fatty acids (SCFAs), tryptophan, tryptamine, and secondary bile acids; (iii) SCFAs stimulate serotonin synthesis and release by their action on enterochromaffin cells; (iv) influence serotonin metabolism; and (v) promote Tph1 expression and stimulate serotonin synthesis (tryptamine is a ligand for the 5-HT4 receptor (5-HT4R) and secondary bile acids, formed by the gut microbiota). Gut microbiota survival, proliferation and metabolism are influenced by EFAs. Dietary EFAs are converted to their long-chain metabolites by desaturases and elongases. EFAs and their metabolites are necessary for the integrity and function of enterochromaffin cells and gut function. By their action on enterochromaffin cells, EFAs can influence serotonin metabolism. EFAs and their metabolites can minimize TLR3 expression. Similarly, EFAs and their metabolites suppress cGAS-STING pathway and inhibit the production of pro-inflammatory cytokines and thus, inhibit their production. The coagulation by aggressive platelets aggregation can also be suppressed by EFAs and their anti-inflammatory metabolites and thus, prevent thrombotic episodes. EFAs and their metabolites stimulate vagus nerve and thus enhance the production of acetylcholine that has anti-inflammatory actions. EFAs and acetylcholine enhance the production of LXA4, a potent anti-inflammatory bioactive lipid (derived from AA). Thus, EFAs and their metabolites have a plethora of actions that explain their role in post-COVID long haul syndrome. The red arrows indicate pro-inflammatory pathways. The blue arrows indicate the pathways stimulated by cytokines that are pro-inflammatory in naure. The green arrows indicate the metabolism of dietary EFAs to their long-chain metabolites and action on enterocytes and their beneficial actions
Scheme showing potential interaction between EFAs and their metabolites and serotonin. Indoles are synthesized from tryptophan by gut microbiota that express tryptophanase (TP). Indoles are cytoprotective molecules. Indolepropionic acid (IPA) synthesized by gut microbiota is a neuroprotective substance and binds to the pregnane X receptor (PXR) in intestinal cells, to facilitate mucosal homeostasis. IPA absorbed from the gut is transferred to the brain to prevent β-amyloid fibril formation. Tryptophan is metabolized to indole-3-aldehyde (I3A) by gut microbiota that acts on the aryl hydrocarbon receptor (AhR) in intestinal immune cells. Gut microbiota have the ability to alter the expression of serotonin-related genes and thus, alters its (serotonin) biosynthesis. Gut microbiota (i) directly act on enterochromaffin (EC) cells to increase colonic tryptophan hydroxylase 1 (Tph1) expression and promote serotonin synthesis; (ii) alter host by direct action or through their metabolites, short chain fatty acids (SCFAs), tryptophan, tryptamine, and secondary bile acids; (iii) SCFAs stimulate serotonin synthesis and release by their action on enterochromaffin cells; (iv) influence serotonin metabolism; and (v) promote Tph1 expression and stimulate serotonin synthesis (tryptamine is a ligand for the 5-HT4 receptor (5-HT4R) and secondary bile acids, formed by the gut microbiota). Gut microbiota survival, proliferation and metabolism are influenced by EFAs. Dietary EFAs are converted to their long-chain metabolites by desaturases and elongases. EFAs and their metabolites are necessary for the integrity and function of enterochromaffin cells and gut function. By their action on enterochromaffin cells, EFAs can influence serotonin metabolism. EFAs and their metabolites can minimize TLR3 expression. Similarly, EFAs and their metabolites suppress cGAS-STING pathway and inhibit the production of pro-inflammatory cytokines and thus, inhibit their production. The coagulation by aggressive platelets aggregation can also be suppressed by EFAs and their anti-inflammatory metabolites and thus, prevent thrombotic episodes. EFAs and their metabolites stimulate vagus nerve and thus enhance the production of acetylcholine that has anti-inflammatory actions. EFAs and acetylcholine enhance the production of LXA4, a potent anti-inflammatory bioactive lipid (derived from AA). Thus, EFAs and their metabolites have a plethora of actions that explain their role in post-COVID long haul syndrome. The red arrows indicate pro-inflammatory pathways. The blue arrows indicate the pathways stimulated by cytokines that are pro-inflammatory in naure. The green arrows indicate the metabolism of dietary EFAs to their long-chain metabolites and action on enterocytes and their beneficial actions
&
lt;strong>Features of Long-COVID
Long-COVID presents a complex array of immune dysregulation, biochemical alterations, and systemic manifestations. Studies have identified elevated levels of non-conventional monocytes, dysregulated cytokine profiles, and persistent viral antibodies in long-COVID patients. These individuals also exhibit abnormalities in the hypothalamic-pituitary-adrenal axis, suggesting an intricate interplay between immune, endocrine, and neurological systems in this syndrome.
Further characterization of immune responses in long-COVID reveals an increased expression of MHC class II molecules and alterations in T cell populations, particularly CD4+ cells. Dysfunctional T cell responses, along with elevated levels of specific antibodies and pro-inflammatory mediators, contribute to the chronic inflammatory state observed in long-COVID.
Serotonin Deficiency in Long-Haul Syndrome
Recent research has unveiled a potential link between serotonin deficiency and the neuropsychiatric symptoms observed in long-COVID. Serotonin, primarily synthesized in the gut, plays a crucial role in mood regulation, cognition, and neurological functions. Reduced plasma serotonin levels in long-COVID patients have been attributed to various factors, including altered tryptophan metabolism, platelet dysfunction, and increased monoamine oxidase activity.
The impact of serotonin deficiency extends beyond neurological symptoms, affecting immune regulation and inflammatory pathways. Serotonin receptors are expressed on immune cells, influencing cytokine production, T cell function, and inflammatory responses. The dysregulation of serotonin signaling pathways due to viral interference or metabolic disruptions may contribute to the immune dysregulation and chronic inflammation observed in long-COVID.
The Role of Essential Fatty Acids (EFAs) in Long-COVID
This
COVID-19 News report covers a hypothesis proposed by a researcher from the Indian Institute of Technology-Hyderabad-India that proposes that essential fatty acids (EFAs) could prevent and ameliorate long covid issues through a variety of ways.
Metabolism of EFAs
Essential fatty acids (EFAs) play a fundamental role in immune regulation, cellular signaling, and neurotransmitter synthesis. Linoleic acid (LA) and alpha-linolenic acid (ALA), classified as EFAs, undergo enzymatic conversions to produce bioactive metabolites such as arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). These metabolites are critical for inflammation resolution, tissue repair, and immune modulation.
The metabolism of EFAs involves desaturases and elongases, enzymes essential for converting dietary EFAs into their long-chain metabolites. Deficiencies or imbalances in EFAs, often exacerbated by dietary factors or genetic polymorphisms, can lead to dysregulated immune responses and compromised tissue healing. The insufficient formation of anti-inflammatory metabolites from EFAs, such as prostaglandins, resolvins, and protectins, contributes to sustained inflammation and immune dysfunction in long-COVID.
EFAs and Serotonin Regulation
Beyond their immunomodulatory roles, EFAs influence serotonin synthesis, transport, and receptor function. Arachidonic acid (AA) and docosahexaenoic acid (DHA) play pivotal roles in serotonin metabolism within the central nervous system (CNS). Alterations in EFA levels can disrupt serotonin homeostasis, impacting mood, cognition, and neurological processes. Restoring EFA balance through dietary interventions or supplementation may alleviate serotonin-related symptoms in long-COVID patients, offering a potential avenue for therapeutic intervention.
EFAs in COVID-19 Pathophysiology
Previous studies suggest that EFAs, especially AA and EPA, possess antiviral properties and modulate immune responses crucial for combating SARS-CoV-2 infection. The dysregulation of EFAs during viral infections exacerbates inflammatory cascades, contributing to the severity of COVID-19 and potentially predisposing individuals to long-term complications. The balance between pro-inflammatory and anti-inflammatory eicosanoids derived from EFAs influences the magnitude and duration of immune responses, impacting disease outcomes.
Gut Microbiota Interactions
EFAs also play a crucial role in modulating gut microbiota composition and activity. The bidirectional relationship between EFAs and gut microbes influences immune function, inflammation, and neurotransmitter metabolism. Short-chain fatty acids (SCFAs) produced by gut bacteria from EFAs contribute to immune regulation, gut barrier integrity, and metabolic processes. Dysbiosis, characterized by an imbalance in gut microbial communities, is associated with chronic inflammation and autoimmune disorders, potentially exacerbating long-COVID symptoms.
Therapeutic Implications and Conclusion
Given the multifaceted involvement of EFAs in immune regulation, neurotransmitter function, and gut microbiota interactions, targeted interventions are warranted in managing long-COVID. Strategies focusing on optimizing EFA intake, enhancing metabolic conversions to anti-inflammatory metabolites, and restoring gut microbial balance may mitigate long-term sequelae and improve outcomes in individuals recovering from COVID-19 or post-vaccination complications.
Future research should explore the specific mechanisms linking EFAs to immune dysregulation, serotonin deficiency, and gut microbiota alterations in long-COVID. Clinical trials evaluating the efficacy of EFA supplementation or dietary interventions in mitigating long-COVID symptoms are essential for establishing evidence-based therapeutic approaches. By addressing the intricate interplay between EFAs, immune responses, and neurological pathways, clinicians can develop targeted strategies to support long-COVID patients and improve their overall well-being.
The hypothesis was published in the peer reviewed journal: Lipids in Health and Disease.
https://lipidworld.biomedcentral.com/articles/10.1186/s12944-024-02090-4
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
