Evolution of SARS-CoV-2 Highlights the Interplay Between Mitophagy and Immune Responses
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 23, 2024 1 year, 1 week, 13 hours, 48 minutes ago
Medical News: The global COVID-19 pandemic brought the virus SARS-CoV-2 into the spotlight, revealing its ability to infect millions and rapidly evolve new variants. Scientists worldwide have been studying how this virus adapts and interacts with the human immune system to evade detection and thrive. A recent study led by researchers from the University of -USA sheds light on a critical aspect of this viral evolution: the interplay between mitophagy, immune signaling, and the evolutionary strategies of SARS-CoV-2.
 Graphical Abstract: Pressure to evade cell-autonomous innate sensing reveals interplay between mitophagy, IFN signaling, and SARS-CoV-2 evolution
What Is Mitophagy and Why Does It Matter
Graphical Abstract: Pressure to evade cell-autonomous innate sensing reveals interplay between mitophagy, IFN signaling, and SARS-CoV-2 evolution
What Is Mitophagy and Why Does It Matter
Mitophagy is a specialized form of autophagy - a process where cells remove damaged components - that specifically targets mitochondria, the powerhouses of cells. This process is vital for maintaining cellular health and regulating immune responses. The research team found that mitophagy has a direct impact on the body’s ability to respond to viral infections like SARS-CoV-2.
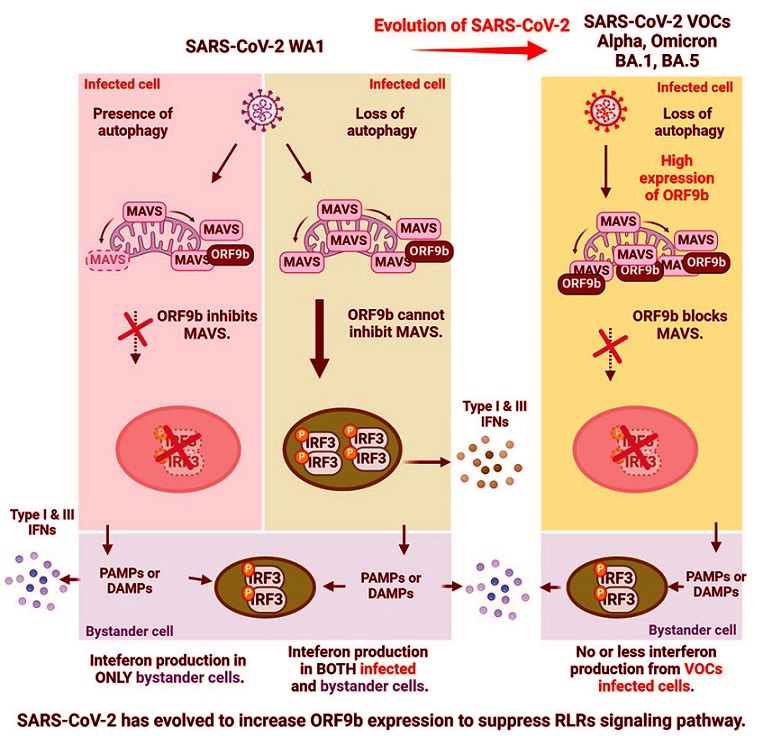
Using various laboratory models, the scientists demonstrated how mitophagy genes regulate mitochondrial antiviral signaling protein (MAVS). This protein is crucial for initiating interferon (IFN) responses, the body's first line of defense against viruses. SARS-CoV-2 has evolved mechanisms to suppress these immune responses, particularly by utilizing a viral protein called ORF9b. This
Medical News report delves deeper into how the virus leverages these pathways to ensure its survival and replication.
How SARS-CoV-2 Exploits Cellular Pathways
One of the most striking findings of this study was that the virus uses ORF9b to suppress MAVS activity. MAVS is critical for activating the production of IFNs, which signal neighboring cells to prepare defenses against viral invasion. By antagonizing MAVS, SARS-CoV-2 can delay the immune response, providing the virus with a window to replicate unchecked.
The researchers also uncovered that autophagy genes - especially those involved in mitophagy - help regulate basal levels of MAVS. When mitophagy genes were depleted, MAVS levels increased, resulting in heightened immune signaling that could block viral replication. This discovery suggests that SARS-CoV-2’s reliance on ORF9b to inhibit MAVS is an evolutionary strategy to overcome the host’s defenses.
Key Findings from the Study
The study provided detailed insights into how SARS-CoV-2 interacts with the host immune system:
-Role of Mitophagy in Immune Regulation:
Mitophagy genes like ATG7, ATG9A, and OPTN were shown to suppress excessive activation of MAVS.
Loss of these genes led to increased MAVS levels, which overrode the effects of ORF9b.
-Evolution of ORF9b Expression:
Variants of SARS-CoV-2, includin
g Alpha and Omicron, express higher levels of ORF9b compared to ancestral strains.
This increased expression enhances the virus’s ability to suppress immune signaling, ensuring its replication even in the presence of robust immune responses.
Mechanisms of Immune Evasion:
By co-localizing with MAVS on mitochondria, ORF9b effectively blocks IFN activation in infected cells.
However, in cells with reduced mitophagy, the heightened MAVS levels can overcome this blockade, restoring immune function.
Implications for Emerging Variants:
Recent variants, including Omicron, have evolved additional strategies to evade immune detection by increasing ORF9b expression and modifying other immune pathways.
The Bigger Picture
These findings highlight the intricate dance between host immune defenses and viral evasion strategies. Mitophagy emerges as a double-edged sword: while it maintains cellular homeostasis, it also creates a vulnerability that SARS-CoV-2 exploits. The evolution of increased ORF9b expression in newer variants underscores the virus’s ability to adapt to host defenses, ensuring its survival in a changing immune landscape.
The researchers also noted that SARS-CoV-2’s strategies extend beyond mitophagy and MAVS. By analyzing various viral proteins, they identified how additional components interact with host cellular machinery to suppress immune responses. These multi-faceted approaches illustrate the complexity of viral adaptation.
Experimental Insights
The researchers employed a range of experimental models, including Calu-3 human respiratory cells, to dissect the roles of autophagy and mitophagy genes.
Key experiments demonstrated:
-Gene Depletion Studies:
Knocking down mitophagy-related genes, such as ATG7 and ATG9A, significantly increased MAVS levels, which activated stronger IFN responses. This response was sufficient to block viral replication in many cases.
-Drug Treatments:
Using inhibitors of autophagy pathways showed similar results, confirming the role of mitophagy in regulating immune responses.
-Variant Analysis:
Comparing different SARS-CoV-2 variants revealed that more recent strains, such as Omicron, expressed higher levels of ORF9b, making them more adept at evading immune detection.
These insights were further validated using human airway models, including primary bronchial and nasal epithelial cells, as well as induced pluripotent stem cell-derived alveolar cells. Across all models, the findings consistently pointed to the critical role of mitophagy in controlling immune responses.
Future Directions and Applications
Understanding how SARS-CoV-2 exploits mitophagy and immune signaling opens new avenues for therapeutic interventions. Potential strategies include:
-Targeting ORF9b:
Developing small molecules or biologics to inhibit ORF9b’s interaction with MAVS could restore effective immune signaling.
-Enhancing MAVS Activity:
Boosting MAVS function, either through gene therapy or pharmacological agents, could counteract the virus’s suppressive effects.
-Broader Immune Modulation:
Therapies that broadly enhance IFN responses, such as recombinant IFNs or STING agonists, may provide additional protection against SARS-CoV-2 and other viral infections.
Additionally, this research underscores the importance of monitoring viral evolution beyond the spike protein. As shown in this study, accessory proteins like ORF9b play pivotal roles in immune evasion and are crucial targets for future research.
Conclusions From the Study
The study, conducted by a multidisciplinary team at the University of Pennsylvania, reveals that mitophagy plays a crucial role in regulating innate immune responses. SARS-CoV-2’s evolution to express higher levels of ORF9b highlights the selective pressure exerted by host immune defenses. These adaptations ensure the virus’s survival even in the presence of strong immune responses.
Furthermore, the findings underscore the need for a comprehensive approach to studying viral evolution. By focusing on both structural changes in the virus and its interactions with host cellular pathways, researchers can develop targeted therapies that disrupt the virus’s ability to evade immune detection while preserving host cellular function.
The study findings were published in the peer-reviewed journal: Cell Reports.
https://www.cell.com/cell-reports/fulltext/S2211-1247(24)01466-9
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/how-sars-cov-2-evades-the-human-immune-system-in-its-evolutionary-journey
https://www.thailandmedical.news/news/sars-cov-2-orf9b-protein-activates-mark2-a-key-enzyme-in-cells
https://www.thailandmedical.news/news/cullin-5-cul5-complex-offers-new-hope-for-covid-19-treatment-by-disabling-orf9b-proteins
https://www.thailandmedical.news/news/shandong-university-study-uncovers-that-sars-cov-2-orf9b-antagonizes-type-i-and-iii-interferons-by-targeting-components-of-signaling-pathways
https://www.thailandmedical.news/articles/coronavirus
