Exploring The Within-Host Evolution Of SARS-CoV-2 - Insights Into Transmission Dynamics And Mutational Outcomes
Nikhil Prasad Fact checked by:Thailand Medical News Team Feb 25, 2024 1 year, 9 months, 3 weeks, 6 days, 10 hours, 12 minutes ago
COVID-19 News: The global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since late 2019 has led to a formidable public health crisis. The ongoing evolution of the virus, marked by the emergence of various variants of concern (VOC), presents significant challenges to public health efforts worldwide. Understanding the within-host dynamics of SARS-CoV-2, including the transmission probabilities of de novo mutations, is crucial for predicting the emergence of future variants. In this
COVID-19 News report, we delve into the complexities of SARS-CoV-2 evolution within infected individuals, exploring the survival and transmission probabilities of novel mutations.
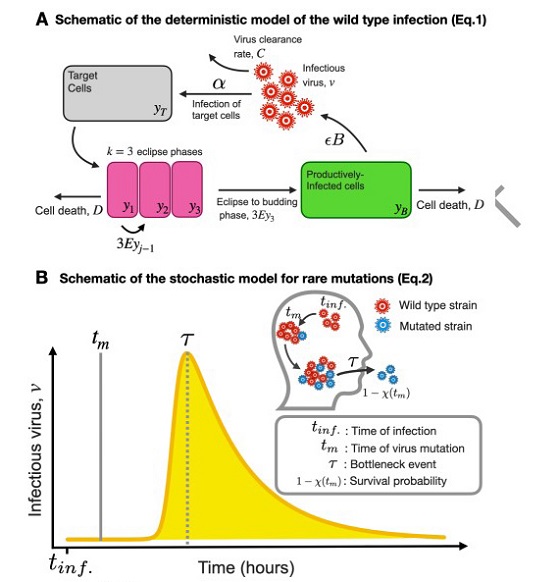 Schematic of the deterministic model of the wildtype infection and Schematic of the stochastic model for rare mutations
Evolutionary Context and Current Landscape
Schematic of the deterministic model of the wildtype infection and Schematic of the stochastic model for rare mutations
Evolutionary Context and Current Landscape
The rapid evolution of SARS-CoV-2 has been facilitated by its substantial genetic diversity, despite a relatively low mutation rate for a viral pathogen. The sheer magnitude of infections, exceeding 750 million cumulative cases globally, has allowed for the accumulation of genetic changes within the virus. This study focuses on the within-host evolution, emphasizing the need for a comprehensive model that incorporates both evolutionary dynamics and transmission probabilities.
Methodology: Stochastic Transmission-Bottleneck Model
To shed light on the within-host evolution of SARS-CoV-2, researchers from York University and Western University in Canada applied a stochastic transmission-bottleneck model. This model takes into account the mutation rate per site replication and within-host parameter estimates for symptomatic SARS-CoV-2 infections. By considering bottleneck size and selection coefficients, the study aims to predict the survival probability of de novo mutations and their subsequent transmission to new hosts.
Predictions and Insights
The study reveals intriguing findings regarding the transmission probabilities of mutations affecting various viral traits. Notably, mutations influencing the per-target-cell attachment rate, associated with fusogenicity and ACE2 binding, demonstrate similar transmission probabilities to mutations affecting viral load clearance linked to humoral evasion. However, mutations affecting the eclipse rate, tied to cellular metabolic processes and viral budding, are highly favored relative to other traits examined.
Moreover, mutations leading to reduced removal rates of infected cells, associated with innate immune evasion, show limited transmission advantage compared to mutations leading to humoral evasion. Despite mutations affecting humoral evasion being more easily transmitted when they occur, the study suggests that mutations influencing innate immune evasion may be more readily generated.
Validation with Known Mutations
The researchers validate their predictions by examining previously characterized mutations in circulating SARS-CoV-2 str
ains. This approach provides a null model for SARS-CoV-2 mutation rates and offers insights into which aspects of viral life history are more likely to evolve successfully, despite the challenges posed by low mutation rates and repeated transmission bottlenecks.
N439K mutation: One example of a known mutation is the N439K mutation in the spike protein of SARS-CoV-2. This mutation has been associated with enhanced ACE2 binding affinity, potentially leading to increased viral attachment to target cells. Additionally, the N439K mutation has also been linked to humoral immune escape, allowing the virus to evade neutralizing antibodies.
In the context of the study's findings, mutations such as N439K, which enhance both ACE2 binding affinity and humoral immune escape, are predicted to have similar transmission probabilities to mutations affecting viral load clearance associated with humoral evasion. This prediction aligns with the observed behavior of the N439K mutation, as it demonstrates a dual advantage in terms of viral attachment and immune evasion, potentially facilitating its transmission to new hosts.
By validating the study's predictions with known mutations like N439K, researchers can gain confidence in the model's ability to accurately assess the transmission probabilities of de novo mutations. This validation process helps refine the model and enhances its utility in predicting the emergence and spread of future SARS-CoV-2 variants, ultimately aiding in the development of targeted interventions and public health strategies.
Here are more examples of known mutations in circulating SARS-CoV-2 strains and how they align with the predictions made by the study:
D614G Mutation: This mutation in the spike protein of SARS-CoV-2 emerged early in the pandemic and quickly became the dominant variant globally. The D614G mutation is associated with increased viral infectivity and transmissibility. In the context of the study's findings, mutations that enhance viral attachment and replication, such as D614G, would be predicted to have higher transmission probabilities due to their ability to more efficiently infect host cells and spread between individuals.
E484K Mutation: The E484K mutation is located in the receptor-binding domain (RBD) of the spike protein and has been associated with immune escape, particularly against neutralizing antibodies generated by prior infection or vaccination. Mutations like E484K, which confer humoral immune evasion, are predicted to have transmission advantages according to the study's model. This aligns with observations of variants containing the E484K mutation, such as the Beta and Gamma variants, exhibiting increased transmissibility despite partial immunity from prior infection or vaccination.
L452R Mutation: Found in the spike protein, the L452R mutation has been associated with increased infectivity and resistance to neutralizing antibodies. This mutation, like others that enhance viral attachment and immune escape, is predicted to have higher transmission probabilities based on the study's model. Variants containing the L452R mutation, such as the Delta variant, have demonstrated heightened transmissibility and have rapidly spread to become dominant in many regions worldwide.
N501Y Mutation: The N501Y mutation, located in the RBD of the spike protein, enhances the binding affinity of the virus to the ACE2 receptor, potentially increasing viral infectivity. Variants carrying the N501Y mutation, such as the Alpha variant, have shown increased transmissibility compared to earlier strains. In the study's framework, mutations that improve viral attachment, such as N501Y, are expected to have higher transmission probabilities, contributing to the spread of these variants.
These examples illustrate how known mutations in circulating SARS-CoV-2 strains align with the predictions made by the study regarding transmission probabilities. By examining the phenotypic effects of these mutations and their impact on viral fitness, researchers can validate the model's predictions and gain insights into the factors driving the emergence and spread of novel variants.
Discussion: Pathogen-Host Arms Race
The article delves into the ongoing conflict between the host and the pathogen, creating an arms race that influences the evolution of SARS-CoV-2. The study's stochastic model explores the fate of both neutral and advantageous mutations, providing a nuanced understanding of the interplay between within-host dynamics and transmission probabilities.
Insights into Within-Host Dynamics
The within-host model is grounded in severe SARS-CoV-2 infections, primarily focusing on the upper respiratory tract. While acknowledging the limitations, the study highlights the importance of future research considering spatial inhomogeneities and spatially dependent bottleneck dynamics, particularly for a comprehensive understanding of SARS-CoV-2 evolution within the entire respiratory tract.
Implications for Public Health and Future Research
As the world grapples with the ongoing threat of SARS-CoV-2 evolution, this study underscores the need for a full model that incorporates both within-host evolutionary dynamics and transmission probabilities. The findings provide valuable insights for predicting the emergence of new variants and informing targeted interventions. Future research directions may include considering the implications of spatial dynamics and exploring the within-host evolution during prolonged infections.
Conclusion
In conclusion, this article explores the within-host evolution of SARS-CoV-2, unraveling the intricate dynamics that govern the survival and transmission probabilities of de novo mutations. The study's predictions offer a valuable framework for understanding the factors influencing the emergence of novel variants, contributing to our collective efforts in managing and mitigating the impact of the ongoing global health crisis.
The study findings were published in the peer reviewed journal: Virus Evolution (Oxford Journals).
https://academic.oup.com/ve/advance-article/doi/10.1093/ve/veae006/7612085
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
