Extracellular Histones And Procoagulant Extracellular Vesicles Drive The Prothrombotic State In COVID-19
Nikhil Prasad Fact checked by:Thailand Medical News Team May 30, 2024 1 year, 6 months, 3 weeks, 5 days, 21 hours, 55 minutes ago
COVID-19 News: The COVID-19 pandemic has presented the medical community with a multitude of challenges, one of the most significant being the development of a prothrombotic state in patients. This state leads to severe complications such as venous thromboembolism (VTE) despite the use of thromboprophylaxis. Understanding the underlying mechanisms of this condition is crucial for developing effective treatments.
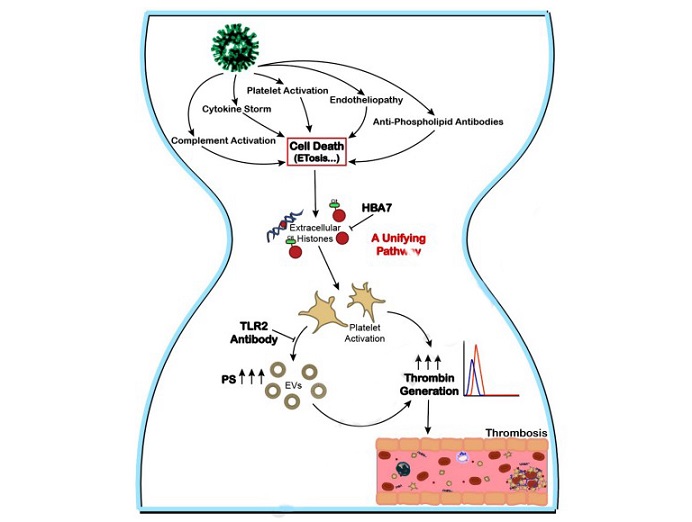 Extracellular histones, a proposed targetable unifying mechanism driving the thrombo-inflammatory state of COVID-19. Proposed mediators of thrombosis in COVID-19 include complement activation, cytokine storm, platelet activation, endotheliopathy, and antiphospholipid antibodies. All of these processes can induce cell death pathways, including the release of extracellular traps (ETosis), resulting in elevation of extracellular histones. Our data suggest that high levels of extracellular histones in COVID-19 plasma trigger platelet prothrombotic activity via TLR2, resulting in the generation of platelet-derived extracellular vesicles (EVs). The activated platelets and platelet-derived EVs (containing surface exposed phosphatidylserine (PS)) drive thrombin generation and thrombus formation. This unifying prothrombotic pathway can be inhibited by the anti-histone aptamer HBA7. Thus, extracellular histones may represent a targetable unifying mechanism driving the thrombo-inflammatory state of COVID-19
The Enigma of COVID-19-Related Thrombosis
Extracellular histones, a proposed targetable unifying mechanism driving the thrombo-inflammatory state of COVID-19. Proposed mediators of thrombosis in COVID-19 include complement activation, cytokine storm, platelet activation, endotheliopathy, and antiphospholipid antibodies. All of these processes can induce cell death pathways, including the release of extracellular traps (ETosis), resulting in elevation of extracellular histones. Our data suggest that high levels of extracellular histones in COVID-19 plasma trigger platelet prothrombotic activity via TLR2, resulting in the generation of platelet-derived extracellular vesicles (EVs). The activated platelets and platelet-derived EVs (containing surface exposed phosphatidylserine (PS)) drive thrombin generation and thrombus formation. This unifying prothrombotic pathway can be inhibited by the anti-histone aptamer HBA7. Thus, extracellular histones may represent a targetable unifying mechanism driving the thrombo-inflammatory state of COVID-19
The Enigma of COVID-19-Related Thrombosis
COVID-19, caused by the SARS-CoV-2 virus, is characterized by severe inflammation and coagulopathy. The prothrombotic state in COVID-19 patients has been linked to various factors, including endotheliopathy, platelet activation, cytokine storms, neutrophil activation, complement activation, and antiphospholipid antibodies. However, the exact pathway leading to thrombosis has remained elusive.
The Role of Extracellular Histones
A breakthrough in understanding the prothrombotic state in COVID-19 that is covered in this
COVID-19 News report, came with the identification of elevated levels of extracellular histones in the plasma of infected patients. Histones, particularly citrullinated histone H3 (H3Cit), are released during cell death processes such as apoptosis, necrosis, and extracellular trap formation (ET). These histones have been found to activate platelets, leading to increased thrombin generation and thrombosis.
Investigating the Mechanism
Researchers from the University of Iowa, Vanderbilt University Medical Center, and the VA Medical Center conducted a study to explore this mechanism further. They utilized blood samples from an early clinical trial of hospitalized COVID-19 patients and recruited healthy subjects as controls. The study aimed to measure the procoagulant and prothrombotic potential of circulating extracellular histones and extracellular vesicles (EVs) in COVID-19 patients.
&l
t;strong>Methods
The researchers measured various parameters in the plasma samples, including levels of citrullinated histone H3, cell-free DNA, nucleosomes, and EVs. They assessed platelet prothrombotic activity through thrombin generation potential and platelet thrombus growth. Additionally, they evaluated the thrombin generation potential of EVs in vitro and their enhancement of thrombotic susceptibility in vivo in mice.
Key Findings
-Elevated Plasma Components: COVID-19 patients had significantly higher levels of citrullinated histone H3, cell-free DNA, nucleosomes, and EVs compared to controls.
-Platelet Activation: Plasma from COVID-19 patients promoted platelet activation, platelet-dependent thrombin generation, and thrombus growth under venous shear stress.
-Extracellular Vesicles: Platelet-derived EVs were released in greater quantities in COVID-19 patients, enhancing thrombin generation in vitro and potentiating venous thrombosis in mice in vivo.
Histone-Targeted Therapy
The study found that these prothrombotic effects were inhibited by an RNA aptamer that neutralizes both free and DNA-bound histones. This suggests that extracellular histones and procoagulant EVs drive the prothrombotic state in COVID-19. Therefore, targeting histones could be a promising therapeutic strategy.
The study supports the hypothesis that extracellular histones act as a unifying mechanism driving platelet-dependent EV release and thrombus formation in COVID-19. The elevated plasma levels of histones correlate with disease severity and could serve as prognostic markers of thrombosis.
Conclusion
Extracellular histones, particularly H3Cit, and EVs play a crucial role in the prothrombotic state observed in COVID-19 patients. By promoting platelet activation and thrombin generation, they contribute significantly to the risk of thrombosis. Histone-targeted therapies could provide a novel approach to managing the thrombo-inflammatory state in COVID-19, potentially improving patient outcomes.
The study findings were published in the peer reviewed Journal of Thrombosis and Haemostasis.
https://www.sciencedirect.com/science/article/pii/S1538783624003052
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/covid-19-causes-morphological-changes-of-red-blood-cells-leading-to-microangiopathy-microthrombosis-impaired-blood-flow-tissue-oxygenation
https://www.thailandmedical.news/news/university-of-bristol-study-finds-that-sars-cov-2-induced-platelet-driven-thromboinflammation-contributes-to-thrombosis-in-covid-19-patients
https://www.thailandmedical.news/news/stellenbosch-university-study-finds-that-long-covid-is-caused-by-the-increased-levels-of-six-inflammatory-molecules-triggering-thrombotic-endotheliiti
