Source : Thailand Medical News Dec 19, 2019 5 years, 4 months, 1 week, 19 hours, 14 minutes ago
Research led by Monash University Professor Dr Eric Morand offers the first real hope for the treatment of l
upus, a disease which affects 1.6 million people in the US and more than 5.4 million globally, 89% women and for which there is no cure.
The study findings were published in the New England Journal Of Medicine (NEJM). The results are of an international, three-year, Phase 3 trial of a potential new drug that treats this autoimmune disease (also known as systemic
lupus erythematosus (
SLE)).
Typically,
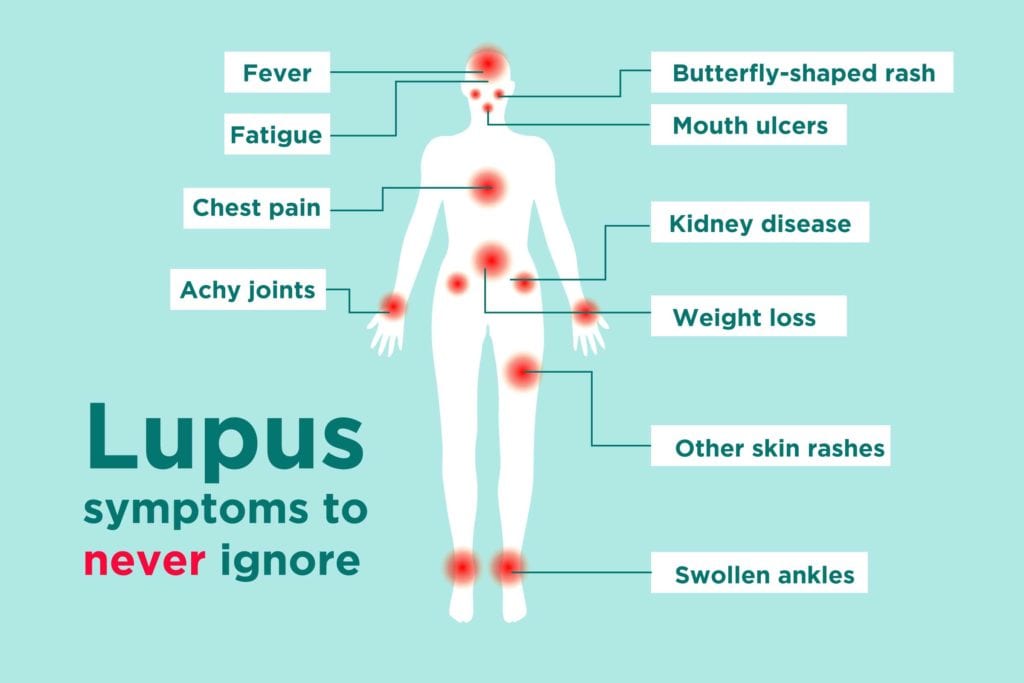
lupus is an autoimmune disease in which the immune system attacks healthy parts of the body.
Professor Morand, who oversaw the global trial in over 360 people with
SLE told
Thailand Medical News, “It is particularly insidious disease as it has a ten-year mortality of 10%, which if you are diagnosed in your early twenties is a terrible outcome."
The clinical trial, called TULIP 2, evaluated
AstraZeneca's
anifrolumab and achieved a statistically-significant and clinically-meaningful reduction in disease activity versus placebo, with both arms receiving standard of care.
Dr Morand has also been key in developing new lupus assessment criteria which because the disease involves a number of organs in the body, can be difficult to both diagnose and monitor.
About 60% and 80% of adults with
SLE show increased interferon-induced genes, which reflect overproduction of the immune protein Type 1 interferon. While previous attempts to block this protein in
lupus have failed, the potential new treatment,
anifrolumab, works by blocking the receptor on all cells in the body, aiming to reverse the triggering of
lupus symptoms.
Dr Morand said that interferon is associated with other autoimmune diseases such as Scleroderma and Sjogren's disease "so there may be potential for using anifrolumab in the treatment of other interferon related diseases as well."
During the TULIP 2 trial, eligible patients received a fixed-dose intravenous infusion of anifrolumab or placebo every four weeks. TULIP 2 assessed the effect of
anifrolumab in reducing disease activity, noting a significant effect in global disease activity measures.
The clinical trial, from 2015 to 2018, involved 362 patients receiving either 300 mg of the drug or a placebo intravenously once every four weeks for 48 weeks. Benefit was measured using a defined clinical assessment of improvement in all organs as well as the number of flare ups (which see the patient experiencing fever, painful or swollen joints, fatigue, rashes or sores or ulcers in the mouth or nose). The volunteers were aged between 18 and 70 and had moderate to severe disease despite standard treatments. Patients with <
strong>SLE typically die of organ failure.
The clinical study found that in 52 weeks after the trial started, significantly more patients on the drug than the placebo had:
-A reduction in overall disease activity in all active organs
-improvement in
lupus skin disease
-A reduction in steroid drug doses
-Reduced annual rate of flares
The positive TULIP 2 trial followed on from the TULIP 1 trial which failed to meet its primary outcome. The second trial, published in the
NEJM, used a different endpoint. "Measurement of treatment response in
SLE has been very problematic and this represents a kind of second breakthrough of this trial," Professor Morand said.
Pharma giant, AstraZeneca will now work with regulators, to bring
anifrolumab, a potential new medicine, to patients. The study was done in collaboration with colleagues in Japan, the UK, the US, France and South Korea.
Reference : Trial of Anifrolumab in Active Systemic Lupus Erythematosus
Eric F. Morand, M.B., B.S., Ph.D., Richard Furie, M.D., Yoshiya Tanaka, M.D., Ph.D., Ian N. Bruce, M.D., Anca D. Askanase, M.D., M.P.H., Christophe Richez, M.D., Ph.D., Sang-Cheol Bae, M.D., Ph.D., M.P.H., Philip Z. Brohawn, M.B.A., Lilia Pineda, M.D., Anna Berglind, Ph.D.,
and Raj Tummala, M.D. December 18, 2019, New England Journal Of Medicine https://www.nejm.org/doi/full/10.1056/NEJMoa1912196 