French Scientists Discover That SARS-CoV-2 Envelope Protein-s PDZ Binding Motif Disrupts Host-s Epithelial Cell-Cell Junction
Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 11, 2024 4 months, 2 weeks, 18 hours, 5 minutes ago
Medical-News: Scientists from several renowned institutions, including Institut Pasteur and Sorbonne Université in France, have made a groundbreaking discovery about the SARS-CoV-2 envelope (E) protein and its role in compromising the human epithelial barrier. This protein, a small yet multifunctional component of the virus, harbors a PDZ Binding Motif (PBM), which acts as a molecular weapon. This
Medical News report delves into the study, which illuminates how the E protein’s PBM significantly affects the virus’s pathogenicity and potential avenues for therapeutic intervention.
 French Scientists Discover That SARS-CoV-2 Envelope Protein’s PDZ Binding Motif Disrupts Host’s
French Scientists Discover That SARS-CoV-2 Envelope Protein’s PDZ Binding Motif Disrupts Host’s
Epithelial Cell-Cell Junction
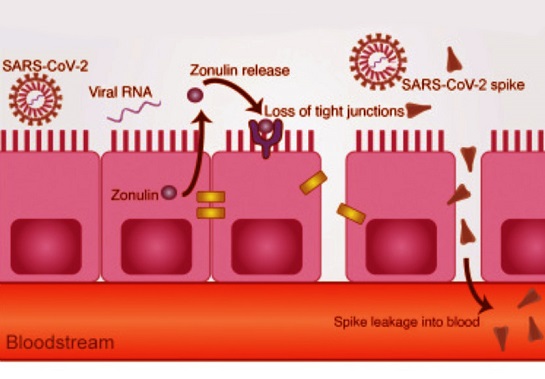
Epithelial cell junctions serve as barriers, shielding underlying tissues from pathogens. SARS-CoV-2, the virus causing COVID-19, has demonstrated an ability to penetrate these defenses, exacerbating airway inflammation and damaging lung tissues. The latest research sheds light on how the PBM in the E protein targets these junctions, leading to severe respiratory complications. This article elaborates on the intricate findings and their implications for combating the virus.
The Study’s Foundation
The collaborative effort involved experts from multiple disciplines, including virology, molecular biology, and histopathology. Researchers focused on the PBM’s contribution to SARS-CoV-2’s virulence using advanced genetic engineering techniques. They created recombinant mutant viruses with either deleted or altered PBMs to evaluate their impact on viral fitness and pathogenicity. The study employed in vitro cell models and in vivo hamster models to investigate these phenomena comprehensively.
“This study provides critical insights into SARS-CoV-2 mechanisms,” said Dr. Flavio Alvarez of Institut Pasteur, one of the lead authors. “Understanding how the PBM facilitates epithelial barrier disruption could guide future antiviral strategies.”
Dissecting the PBM’s Role
The E protein’s PBM directly interacts with host PDZ domain-containing proteins, which are crucial for maintaining cell junction integrity. By disrupting these interactions, the PBM compromises epithelial cell-cell junctions, leading to increased permeability and tissue damage.
-In Vitro Observations: In laboratory cell models, the viruses with intact PBMs showed rapid replication and more pronounced cytopathic effects. Conversely, PBM-deficient mutants exhibited delayed replication and reduced ability to damage cell monolayers.
-In Vivo Findings: When tested on golden hamsters, viruses lacking PBMs caused significantly milder symptoms. Animals infected with these mutants displayed less weight loss, reduced lung inflammation, and lower viral loads compared to those infected with the wild-type vir
us. Additionally, histopathological analyses revealed that PBM-deficient viruses caused less structural damage to lung tissues.
Molecular Interactions: The PBM’s ability to bind tight junction proteins like ZO-1 - essential for epithelial barrier integrity - emerged as a pivotal factor in its pathogenicity. Mutant viruses with altered PBMs showed weakened interactions with ZO-1, leading to better preservation of epithelial structures.
Inflammatory Pathways and Immune Response
RNA sequencing of lung tissues from infected hamsters provided insights into the immune and inflammatory pathways influenced by the PBM. The findings revealed that the PBM amplifies the activation of key inflammatory pathways, including NF-κB and TNF signaling. These pathways contribute to the excessive immune response observed in severe COVID-19 cases.
Interestingly, PBM-deficient viruses induced milder inflammatory responses. Cytokine levels, including IL-6 and TNF-α, were significantly lower in hamsters infected with these mutants. This observation suggests that the PBM plays a pivotal role in driving the hyperinflammatory state associated with SARS-CoV-2 infection.
Neuroinvasion and Epithelial Specificity
One intriguing aspect of the study was its exploration of the PBM’s role in neuroinvasion. Despite previous concerns about the virus’s ability to cross the blood-brain barrier, the researchers found no significant differences in viral loads across brain regions in hamsters infected with either wild-type or PBM-deficient viruses. This suggests that the PBM primarily targets epithelial barriers rather than neurological structures.
Potential Therapeutic Implications
The discovery of the PBM’s critical role in epithelial barrier disruption highlights its potential as a therapeutic target. Inhibiting the PBM-host protein interactions could prevent the virus from compromising epithelial junctions, thereby mitigating its pathogenic effects. Moreover, this approach may reduce the severity of inflammatory responses, addressing one of the major causes of morbidity in COVID-19 patients.
Conclusions
The study conclusively demonstrates that the SARS-CoV-2 E protein’s PBM is a key virulence factor. By disrupting epithelial cell-cell junctions and amplifying inflammatory pathways, the PBM exacerbates the virus’s pathogenicity. However, its limited role in neuroinvasion underscores its tissue-specific effects.
These findings open new avenues for therapeutic interventions. Targeting the PBM could preserve epithelial integrity and reduce inflammation, offering a dual benefit in managing COVID-19. As researchers continue to unravel the molecular intricacies of SARS-CoV-2, the E protein’s PBM stands out as a promising focal point for antiviral strategies.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2024.12.10.627528v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-envelope-protein-down-regulates-tight-junctional-proteins-compromising-the-integrity-of-the-airway-epithelial-barrier
https://www.thailandmedical.news/news/covid-19-news-french-study-finds-that-sars-cov-2-envelop-protein-has-a-carboxy-terminal-that-contains-a-pdz-binding-motif-that-is-pathogenetic
https://www.thailandmedical.news/news/sars-cov-2-e-protein-triggers-neuron-cell-death-via-calcium-release
https://www.thailandmedical.news/news/scientists-discover-an-amyloidogenic-fragment-in-sars-cov-2-envelope-protein-that-promotes-serum-amyloid-a-misfolding-and-fibrillization
https://www.thailandmedical.news/news/unraveling-the-intricacies-of-sars-cov-2-evolution-the-role-of-envelope-protein-mutation-t9i-in-autophagy-resistance
