French Study Discovers That SARS-CoV-2 Uses Tunneling Nanotubes (TNT) To Spread Between Permissive Cells And Non-Permissive Neuronal Cells
Source: Medical News - SARS-CoV-2 Uses Tunneling Nanotubes (TNT) Nov 24, 2021 3 years, 4 months, 3 weeks, 3 days, 1 hour, 6 minutes ago
A new study by researchers from Institut Pasteur-France has found that SARS-CoV-2 uses tunneling nanotubes (TNT) to spread between permissive cells and non-permissive neuronal cells.
Although SARS-CoV-2 entry into host cells is mediated by the binding of its spike glycoprotein to the angiotensin-converting enzyme 2 (ACE2) receptor which are highly expressed in several organs, these receptors have a low presence in the human brain and also in other organs and tissues which have been found to be affected by the virus.
To date the mechanism through which SARS-CoV-2 infects neurons is not understood.
However, it has been known that Tunneling nanotubes (TNTs) ie actin-based intercellular conduits that connect distant cells, allow the transfer of cargos, including viruses.
The study team explored the neuroinvasive potential of SARS-CoV-2 and whether TNTs are involved in its spreading between cells
in vitro.
The study findings show that neuronal cells, not permissive to SARS-CoV-2 through an exocytosis/endocytosis dependent pathway, can be infected when co-cultured with permissive infected epithelial cells. SARS-CoV-2 induces TNTs formation between permissive cells and exploits this route to spread to uninfected permissive cells in co-culture.
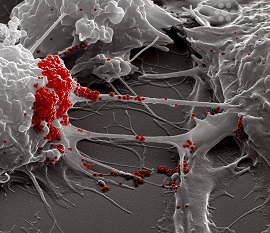
Detailed correlative Cryo-electron tomography used in the study revealed that SARS-CoV-2 is associated with the plasma membrane of TNTs formed between permissive cells and virus-like vesicular structures are inside TNTs established both between permissive cells and between permissive and non-permissive cells.
The study findings highlight a potential novel mechanism of SARS-CoV-2 spreading which could serve as route to invade non-permissive cells and potentiate infection in permissive cells.
The study findings were published in a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.11.15.468633v1
To date, numerous differential neurological manifestations associated with the coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 infection, have been reported.
Despite the fact that acute neurological symptoms may eventually resolve, these symptoms have often been reported to persist during long-COVID. To date, it remains unclear how SARS-CoV-2 gains access to the central nervous system (CNS).
It is known that SARS-CoV-2 binds its spike protein to the host cell’s surface angiotensin-converting receptor 2 (ACE2) to gain entry. ACE2 receptors are found in organs such as the lungs, intestines, liver, kidneys, and heart.
But the expression of ACE2 is low in the human brain, with the exception of certain areas such as the thalamus and choroid plexus.
Still, despite the overall lack of ACE2 receptors in the brain, SARS-CoV-2 has been found to propagate throughout the brain tissues of COVID-19 decedents.
In a past study, researchers have shown a novel mechanism of cell-to-cell communication by spreading different amyloid aggregates between the CNS and from the peripheral lymphoid system cells to neurons via TNTs.
https://pubmed.ncbi.nlm.nih.gov/19198598/<
;/a>
https://pubmed.ncbi.nlm.nih.gov/27550960/
https://pubmed.ncbi.nlm.nih.gov/31625188/
Tunneling nanotubes or TNTs are dynamic connections between cells that consist of thin membranous conduits rich in actin that form contiguous cytoplasmic bridges between cells over long and short distances. These TNTs are capable of transporting cargo, including viruses, between cells.
Numerous studies have also reported the formation of TNTs or TNT-like structures that have been induced by HIV virus, retroviruses, herpesviruses, influenza A, and the human metapneumovirus to enable the efficient spread of infection to neighboring healthy cells.
https://pubmed.ncbi.nlm.nih.gov/18835599/
https://pubmed.ncbi.nlm.nih.gov/32024778/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3232570/
https://pubmed.ncbi.nlm.nih.gov/30917314/
https://pubmed.ncbi.nlm.nih.gov/18193035/
Hence as a result of these previous observations, the researchers of the current study investigated the TNTs role in the neuroinvasive potential of SARS-CoV-2.
The study team importantly noted that when the virus is transferred through the TNTs, viruses escape neutralizing antibody activity and immune surveillance.
Corresponding author, Dr Chiara Zurzolo from the Département de Biologie Cellulaire at Institut Pasteur told Thailand
Medical News, “Alarmingly these SARS-CoV-2 viruses are also able to invade less permissive cells, such as those lacking the receptor for virus entry. Thus, the viruses favorably induce TNT formation to allow for the spread of virus tropism and pathogenicity.”
In order to understand how the SARS-CoV-2 infects neuronal cells, the study team tested the permissivity of several different cell types to viral infection by the receptor-mediated pathway.
The various cell lines used in this study included human colon epithelial cell lines (Caco-2,), monkey kidney epithelial cell-line (Vero E6), and the human (SH-SY5Y) and murine (CAD) neuronal cells lines.
The study team found that only the epithelial Vero E6 and Caco-2 cells, rather than the neuronal cells, were susceptible to infection by SARS-CoV-2.
However, upon co-culturing, the study team found that the virus was transferred by a cell-to-cell contact-dependent mechanism and actively replicated in the neuronal cells.
The study findings thus established that the SARS-CoV-2 can spread among cells through an exocytosis/endocytosis independent pathway.
Further probing the direct cell-to-cell contact-dependent manner of the viral spread in neuronal cells, the study team tested whether the virus triggered the formation of the TNTs or TNT-like structures in the infected cells and uses these structures to efficiently spread to uninfected cells.
Utilizing confocal microscopy, the study team confirmed that TNTs contribute to the SARS-CoV-2 transmission.
To further validate and identify the nature and structure of the viral particles shared by TNTs and their mechanism of transfer, the study team set up a correlative fluorescence and cryo-electron microscopy and tomography approach (CLEM, cryo-EM, and cryo-ET, respectively).
Significantly, the images revealed viral compartments in TNTs. This included the presence of membranous structures of various sizes resembling double-membrane vesicles inside the tube, between permissive (Vero E6 infected cells) and non-permissive cells (SH-SY5Y mCherry cells).
The study findings further also confirmed that these TNTs facilitate SARS-CoV-2 transmission between the permissive Vero E6 cells through a secretion-independent pathway.
Between these permissive cells, the study team found that the SARS-CoV-2 particles decorating the TNTs’ surface-displayed both an ellipsoidal- and spherical-enveloped morphology with an average diameter ranging from 50 to 100 nanometer (nm), typically of a coronavirus.
Interestingly, here the cryo-EM images revealed SARS-Cov-2 on top of the TNTs, which was unlike the observation in the neuronal cells.
The study findings provide new information on the structure of the viral particles involved in intercellular spreading and provides important information about the mechanism of SARS-CoV-2 infection and transmission in neuronal cells.
In addition, the findings show the remarkable role of the induced TNTs in the viral transmission in both permissive and non-permissive cells, possibly enhancing the efficiency of viral propagation through the body. The study team states this report represents the first evidence that TNTs could be one possible route for the spreading of SARS-CoV-2.
The study team also say that the utilization of TNTs by the SARS-CoV-2 virus could also account for damage in other human host organs and tissues where there are low or no levels of ACE2 receptors.
For the latest
SARS-CoV-2 Research, keep on logging to Thailand Medical News.
