French Study Shows That The Antibiotic Clofoctol Could Be Repurposed To Treat COVID-19
Source: COVID-19 Drugs - Clofoctol May 30, 2022 2 years, 10 months, 3 weeks, 6 days, 3 hours, 30 minutes ago
COVID-19 Drugs: A new study by researchers from the University of Lille-France, the Institut Pasteur de Lille-France, the Center for Infection and Immunity of Lille-France, CNRS, INSERM, and CHU Lille0France has found that the existing antibiotic Clofoctol could be repurposed to treat COVID-19.

Clofoctol is a bacteriostatic antibiotic. It is used in the treatment of respiratory tract and ear, nose and throat infections caused by Gram-positive bacteria. It has been marketed in France till 2005 under the trade name Octofene and in Italy as Gramplus. Clofoctol has been shown to also possess antiviral properties against certain classes of viruses.
The approach of drug repurposing has the advantage of shortening regulatory preclinical development steps especially in dire situations such as the current COVID-19 pandemic where to date they there are no real effective drugs or therapeutics except ineffective and toxic compounds being peddled by those in control of the COVDI-19 narratives.
The
COVID-19 Drugs study team screened a library of drug compounds, already registered in one or several geographical areas, to identify those exhibiting antiviral activity against SARS-CoV-2 with relevant potency. Of the 1,942 compounds tested, 21 exhibited a substantial antiviral activity in Vero-81 cells. Among them, clofoctol, an antibacterial drug used for the treatment of bacterial respiratory tract infections, was further investigated due to its favorable safety profile and pharmacokinetic properties.
Interestingly, the peak concentration of clofoctol that can be achieved in human lungs is more than 20 times higher than its IC50 measured against SARS-CoV-2 in human pulmonary cells. This compound inhibits SARS-CoV-2 at a post-entry step.
The study team demonstrated that, in vivo, clofoctol reduces inflammatory gene expression and lowers pulmonary pathology.
In the study, therapeutic treatment of human ACE2 receptor transgenic mice decreased viral load, reduced inflammatory gene expression and lowered pulmonary pathology.
The antiviral and anti-inflammatory properties of clofoctol, associated with its safety profile and unique pharmacokinetic properties make a strong case for proposing clofoctol as an affordable therapeutic candidate for the treatment of COVID-19 patients.
The study findings were published in the peer reviewed journal: PLOS Pathogens.
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010498
It should be noted that repurposed drugs often have a speedier path to clinical use because they have already been shown to be safe in people.
The study team was led by Dr Sandrine Belouzard and Dr Jean Dubuisson at Pasteur Institute, Lille, France.
Although it is claimed that COVID-19 vaccines reduce hospitalizations and death, they do not control virus transmission, and affordable, effective therapies are urgently needed.
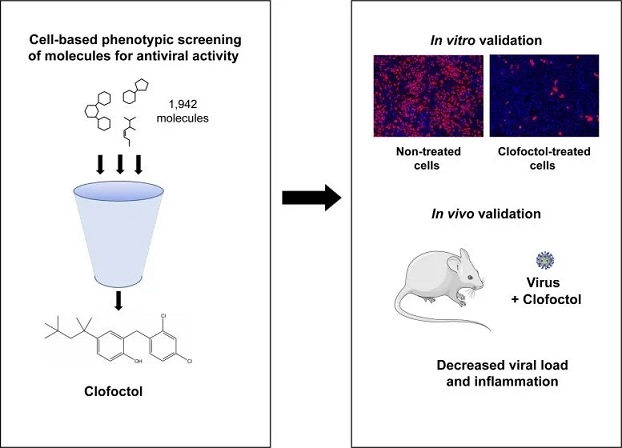 color:#A9A9A9">Clofoctol was identified as a potential antiviral against SARS-CoV-2 following a cell-based phenotypic screening of approximately 2000 drug compounds that have been used or are still used in the clinics. After in vitro validation of the antiviral activity of clofoctol, this compound was confirmed to decrease viral load and inflammation in a humanized mouse model of COVID-19. Credit: Sandrine Belouzard
color:#A9A9A9">Clofoctol was identified as a potential antiviral against SARS-CoV-2 following a cell-based phenotypic screening of approximately 2000 drug compounds that have been used or are still used in the clinics. After in vitro validation of the antiviral activity of clofoctol, this compound was confirmed to decrease viral load and inflammation in a humanized mouse model of COVID-19. Credit: Sandrine Belouzard
Numerous past attempts to repurpose medicines to treat COVID-19 patients have been unsuccessful thus far. In order to identify potential antiviral therapies that are effective against COVID-19, the study team accessed the Apteeus drug library, a collection of 1,942 approved drugs to identify molecules that exhibit antiviral activity against SARS-CoV-2. The team selected clofoctol based on its antiviral potency. They tested their hypothesis by measuring clofoctol’s effects in SARS-CoV-2-infected mice.
The study findings showed that that transgenic mice treated with clofoctol had a decreased viral load, reduced inflammatory gene expression, and lowered pulmonary pathology.
More detailed studies are needed to further understand the drug’s therapeutic potential in SARS-CoV-2 patients as the study was limited by the physiological differences between humans and mice. Additionally, the mice were euthanized only two days after treatment, so longer-term effects remain unknown.
Dr Belouzard told Thailand
Medical News, “The antiviral and anti-inflammatory properties of clofoctol, associated with its safety profile and unique pharmacokinetics make a strong case for proposing clofoctol as an affordable therapeutic candidate for the treatment of COVID-19 patients. Finally, the relatively low cost of this drug suggests that it is a potential clinical option for treatment of COVID-19 patients in resource-poor settings.”
Dr Dubuisson added, “Antivirals targeting SARS-CoV-2 are sorely needed. In this study, we screened a library of drug compounds and identified clofoctol as an antiviral against SARS-CoV-2. We further demonstrated that, in vivo, this compound reduces inflammatory gene expression and lowers pulmonary pathology and decreases viral load.”
The study team is next planning a series of observational clinical trials before initiating a large randomized clinical trial in covering a few centers in Europe.
For the latest on
COVID-19 Drugs, keep on logging to Thailand
Medical News.

