French Study Uncovers That A Short Sequence In The Tail Of SARS-CoV-2 Envelope Protein Controls Accessibility Of Its PDZ Binding Motif To The Cytoplasm
Nikhil Prasad Fact checked by:Thailand Medical News Team Aug 31, 2023 1 year, 7 months, 3 weeks, 21 hours, 26 minutes ago
COVID-19 News: In a collaborative effort, researchers from the Institut Pasteur and Université Paris-France have delved into the intricate molecular mechanisms governing the pathogenicity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus, responsible for the COVID-19 pandemic, has brought the world to its knees as seen in various past
COVID-19 News coverages, sparking unprecedented research endeavors to uncover its secrets.
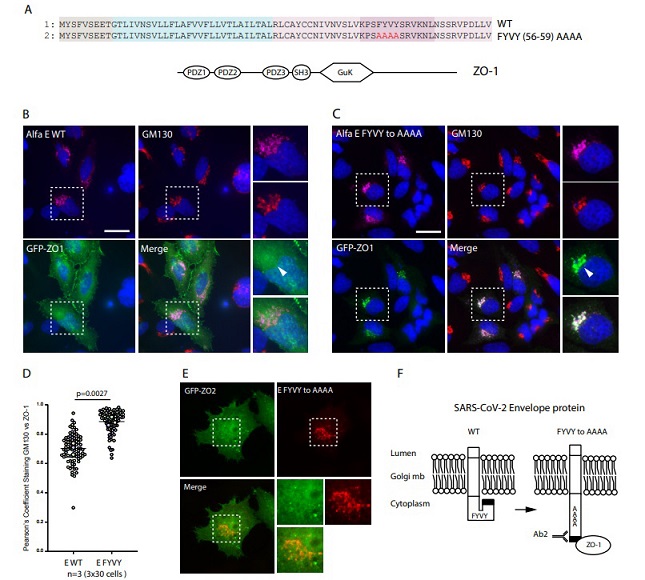 Hela cells were co-transfected with GFP-ZO-1 full length construct (A) and an Alfa tagged E WT(25, 32) encoding plasmid and Golgi localization was quantified using GM130. GFP-ZO-1 full construct exhibits limited co-localization with GM130 (B). Because we previously observed that accessibility to the C-terminal extremity of the E protein was improved by the FYVY sequence mutation, we expressed GFP-ZO-1 together with FYVY to AAAA E mutants in Hela cells (C). In contrast to the WT sequence, the E-FYVY to AAAA mutant robustly recruited ZO-1 protein to the Golgi apparatus (C and D). This Golgi recruitment triggered by the E-FYVY mutant is specific to ZO-1 as it was not observed with construct expressing the full length homologue PDZ domaincontaining protein ZO-2 fused to GFP (E). Taken together, these results indicate that the FYVY sequence increases the exposure of the PDZ binding motif to the cytoplasmic compartment thus regulating the binding of the E PBM to the PDZ domain of host proteins
Hela cells were co-transfected with GFP-ZO-1 full length construct (A) and an Alfa tagged E WT(25, 32) encoding plasmid and Golgi localization was quantified using GM130. GFP-ZO-1 full construct exhibits limited co-localization with GM130 (B). Because we previously observed that accessibility to the C-terminal extremity of the E protein was improved by the FYVY sequence mutation, we expressed GFP-ZO-1 together with FYVY to AAAA E mutants in Hela cells (C). In contrast to the WT sequence, the E-FYVY to AAAA mutant robustly recruited ZO-1 protein to the Golgi apparatus (C and D). This Golgi recruitment triggered by the E-FYVY mutant is specific to ZO-1 as it was not observed with construct expressing the full length homologue PDZ domaincontaining protein ZO-2 fused to GFP (E). Taken together, these results indicate that the FYVY sequence increases the exposure of the PDZ binding motif to the cytoplasmic compartment thus regulating the binding of the E PBM to the PDZ domain of host proteins
One of the latest breakthroughs from these institutions sheds light on a hitherto enigmatic aspect of the virus: the role of a short sequence in the tail of the SARS-CoV-2 envelope protein (E) in controlling the accessibility of its PDZ binding motif (PBM) to the cytoplasm. This discovery offers a new perspective on the virus's interaction with host cells and opens avenues for potential therapeutic interventions.
The Envelope Protein and its Implications in Pathogenicity
At the heart of this study is the SARS-CoV-2 envelope protein (E), a relatively small molecule with big implications for the virus's virulence. The envelope protein not only plays a pivotal role in viral entry into host cells but also orchestrates various aspects of the virus's lifecycle, including replication, assembly of the viral genome, and release of viral particles. This multifaceted protein has been classified as a viroporin, a class of viral proteins capable of forming pores in the membranes of host cells. Viroporins are known for their influence on cellular processes and have become promising targets for antiviral therapies.
Unmasking the PDZ Binding Motif
One of the intriguing features of the envelope protein is its tail sequence, which harbors a PDZ-binding motif (PBM). This PBM is a key player in the virus's interaction with host cells. PDZ domains are found in a variety of host proteins involved in processes critical for viral infection, including cell signaling, metabolism, and immune response. By binding to PDZ domains, the virus can manipulate these host proteins to its advantage, enhancing its replication and dissemination within the host.
&nb
sp;
The Golgi Apparatus and the Puzzle of PBM Presentation
The study team focused on the envelope protein's presence within the Golgi apparatus of host cells. This organelle is central to protein processing and trafficking, and its involvement in the virus lifecycle has long been suspected.
However, the mechanisms underlying the presentation of the PBM to the cytoplasm remained mysterious. To unravel this puzzle, the researchers employed a combination of experimental techniques, including immunofluorescence assays, transfection studies, and molecular analysis.
Revealing the PBM's Elusive Nature
The research revealed an intriguing phenomenon: the PBM in the envelope protein's tail sequence remained elusive to detection by antibodies within the Golgi apparatus. While antibodies recognizing internal epitopes of the envelope protein could detect it at the Golgi apparatus, those targeting the PBM region failed to do so. This enigma prompted the researchers to explore the factors controlling PBM accessibility and presentation to the cytoplasm.
Lysosomal Compartmentalization: A Key to PBM Accessibility
The researchers discovered that lysosomal compartmentalization played a pivotal role in enhancing the accessibility of the envelope protein's carboxy-terminal extremity, which includes the PBM. Lysosomes are cellular structures involved in degradation and recycling processes. The findings showed that the envelope protein's C-terminal region was better detected by antibodies when the protein was directed toward lysosomes. This revelation highlighted the intricate interplay between cellular compartments and viral protein accessibility.
Mutation Unlocks the PBM's Secrets
Further investigation into the sequence of the envelope protein led to a groundbreaking mutation: changing the FYVY sequence to AAAA. This simple alteration led to a remarkable transformation in PBM accessibility and interaction. The mutated protein was better detected by antibodies and exhibited enhanced recruitment of PDZ domain-containing proteins, particularly ZO-1, to the Golgi apparatus. This mutation acted as a key to unlocking the PBM's secrets, shedding light on its conformational regulation and interaction dynamics.
Implications and Future Avenues
This study offers a fresh perspective on the interaction between SARS-CoV-2 and its host cells. By uncovering the mechanisms controlling the accessibility of the PBM, researchers gain deeper insights into the virus's ability to manipulate host proteins and hijack cellular processes. These findings not only contribute to our understanding of SARS-CoV-2 but also provide potential targets for therapeutic interventions.
As researchers continue to probe the intricacies of SARS-CoV-2 and other viruses, the journey towards effective antiviral strategies takes a significant step forward. By deciphering the molecular dance between viral proteins and host cells, scientists pave the way for innovative treatments that could mitigate the impact of viral infections. The collaborative efforts of institutions like Institut Pasteur and Université Paris-France exemplify the global scientific community's dedication to unraveling the mysteries of the microscopic world and its macroscopic consequences.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2023.08.29.555304v1
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
