Nikhil Prasad Fact checked by:Thailand Medical News Team Dec 16, 2024 11 months, 4 weeks, 1 day, 23 hours, 43 minutes ago
Medical News: The SARS-CoV-2 virus has been under relentless scrutiny since the outbreak of the COVID-19 pandemic. Scientists worldwide have focused on understanding the mechanisms that enable the virus to invade human cells. Among the most critical steps in this process is membrane fusion, a mechanism by which the virus merges its lipid envelope with the membrane of a host cell. This essential step allows the viral RNA to enter the host cell and initiate infection. A new study sheds light on the role of a specific region of the viral spike protein, called FP1, in destabilizing membranes and facilitating pore formation during this fusion process.
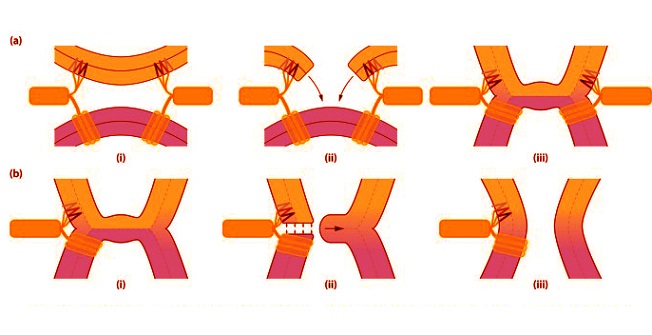 Mechanistic illustration of FP1‐mediated membrane perturbation and its possible role in viral fusion (a) catalysis of hemifusion diaphragm formation: (i) FP1 inserts into the target membrane, locally perturbing its structure. (ii) This perturbation induces rupture of the target membrane, leading to local destabilization. (iii) Membrane reconnection between the viral and target membranes subsequently forms a hemifusion diaphragm. (b) catalyzing fusion pore formation: (i) FP1, positioned laterally to the hemifusion diaphragm, further destabilizes the surrounding lipid bilayer, concentrating its effect at the trilamellar junction. (ii) This destabilization triggers pore formation at the junction. (iii) The hemifusion diaphragm collapses into the membrane, resulting in the formation of a fusion pore.
Mechanistic illustration of FP1‐mediated membrane perturbation and its possible role in viral fusion (a) catalysis of hemifusion diaphragm formation: (i) FP1 inserts into the target membrane, locally perturbing its structure. (ii) This perturbation induces rupture of the target membrane, leading to local destabilization. (iii) Membrane reconnection between the viral and target membranes subsequently forms a hemifusion diaphragm. (b) catalyzing fusion pore formation: (i) FP1, positioned laterally to the hemifusion diaphragm, further destabilizes the surrounding lipid bilayer, concentrating its effect at the trilamellar junction. (ii) This destabilization triggers pore formation at the junction. (iii) The hemifusion diaphragm collapses into the membrane, resulting in the formation of a fusion pore.
Researchers from the Research Institute for Systems Biology and Medicine in Moscow-Russia have delved deep into the workings of FP1, a section of the spike protein’s fusion machinery. Their findings reveal not just how this peptide destabilizes cell membranes but also how it opens up possibilities for novel therapeutic strategies. This
Medical News report provides an in-depth view of their discoveries.
Understanding the Fusion Process
The SARS-CoV-2 spike protein is composed of two primary subunits: S1, which binds to the host cell receptor ACE2, and S2, which mediates membrane fusion. The S2 subunit harbors FP1, a highly hydrophobic peptide critical for the virus’s ability to merge its envelope with the host cell membrane. When the virus binds to a host cell, FP1 inserts itself into the cell membrane, causing significant disruptions. These disruptions play a pivotal role in overcoming the energy barriers required for fusion.
The study focused on FP1, analyzing its interactions with different types of lipid membranes that mimic cellular environments. Researchers used advanced techniques such as electrophysiology, fluorescence spectroscopy, and atomic force microscopy (AFM) to explore how FP1 influences the stability and permeability of membranes.
What the Study Revealed
The researchers synthesized FP1, a 20-amino-acid peptide, to investigate its role in membrane fusion. Through circular dichroism spectroscopy, they observed that FP1 undergoes significant structural changes upon binding to lipid membranes. In aqueous solutions, the peptide remains largely unstructured, but it adopts an alpha-helical structure when it interacts wi
th hydrophobic regions of lipid bilayers.
Experiments conducted on artificial lipid membranes revealed that FP1 facilitates pore formation, a critical step in viral entry. When inserted into a lipid bilayer, FP1 reduces the energy barrier needed for defects to form in the membrane, ultimately leading to pore formation. These pores create pathways for viral RNA to penetrate host cells.
Interestingly, the study demonstrated that the membrane composition plays a crucial role in FP1’s activity. Cholesterol-rich membranes, which resemble those in human cells, showed greater resistance to FP1-induced disruptions. However, even these membranes eventually succumbed to FP1’s destabilizing effects. This dual behavior highlights FP1’s efficiency in adapting to varying membrane environments.
Advanced Tools Shed Light on the Mechanisms
Atomic force microscopy allowed the researchers to measure the mechanical properties of lipid bilayers with remarkable precision. They discovered that FP1 reduces the force required to rupture membranes, indicating that it weakens their structural integrity. These findings were consistent across different lipid compositions, affirming FP1’s potent ability to compromise membrane barriers.
In addition, electrophysiology experiments provided real-time data on how FP1 induces electrical conductance in membranes, a hallmark of pore formation. This technique revealed distinct patterns of membrane disruption, ranging from transient defects to stable conducting pores. Notably, cholesterol-containing membranes exhibited more complex behavior, forming metastable pores that delayed but did not prevent FP1-induced disruptions.
Implications for Antiviral Strategies
The detailed insights into FP1’s role in membrane disruption have significant implications for combating SARS-CoV-2. By targeting FP1 and its interaction with host cell membranes, researchers could develop therapies that block viral entry. Monoclonal antibodies or small molecules designed to inhibit FP1’s activity could serve as potent antiviral agents, preventing the virus from infecting host cells.
Furthermore, FP1’s conserved nature among coronaviruses makes it a promising target for broad-spectrum antiviral therapies. Future vaccines could incorporate FP1 epitopes to elicit robust immune responses, providing protection against a range of coronavirus strains.
Conclusion: Beyond Basic Science
This study highlights the intricate role of FP1 in facilitating SARS-CoV-2 entry into human cells. By destabilizing membranes and promoting pore formation, FP1 emerges as a key player in the viral fusion process. These findings not only deepen our understanding of coronavirus biology but also pave the way for innovative therapeutic approaches.
The implications extend beyond SARS-CoV-2, offering valuable insights into the general mechanisms of viral fusion. As researchers continue to unravel the complexities of viral entry, targeting fusion peptides like FP1 may prove to be a game-changing strategy in the fight against infectious diseases.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.preprints.org/manuscript/202412.1202/v1
For the latest COVID-19 News, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/sars-cov-2-spike-s-fusion-peptide-binds-to-negatively-charged-phospholipids-in-cell-membranes-of-host
https://www.thailandmedical.news/news/breaking-covid-19-news-integrin-alpha5-beta1-contributes-to-cell-fusion-and-inflammation-mediated-by-sars-cov-2-spike-via-rgd-independently
https://www.thailandmedical.news/articles/coronavirus
