Source: Wellcome Trusts Sanger Institute Jul 11, 2018 7 years, 7 months, 2 weeks, 3 days, 15 hours, 4 minutes ago
Genetic variants can increasingly be used to connect patients to treatments as well as uncover new therapeutic targets.
Jeffrey Barrett, formerly of the Wellcome Trust Sanger Institute, described how prioritizing genome-wide association study results has uncovered drugs that could be used to treat inflammatory bowel disease.
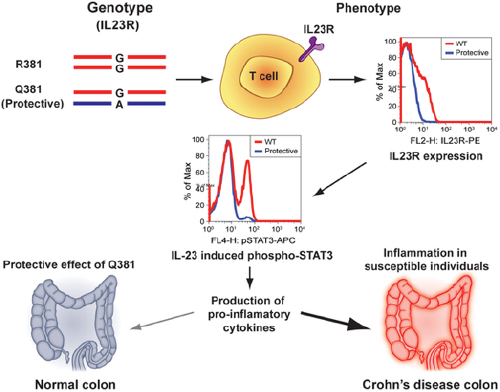
Functional Studies on the IBD Susceptibility Gene IL23R Implicate Reduced Receptor Function in the Protective Genetic Variant R381Q
IBD affects about 1.3 percent of US adults, according to the Centers for Disease Control and Prevention. Barrett, who is now at the
genomics healthcare firm Genomics, said that the condition is debilitating and that over time patients often need surgery,
suggesting that better drugs are needed to control the condition. The genetics of the condition, he further noted, are fairly well studied and could be applied to that effort.
Barrett noted that an anti-TNF drug, introduced in 1998, has treated numerous IBD patients. But while he said it's been beneficial, about 20 percent of IBD patients do not respond to it and about 35 percent lose that response within a year.
The Personalising Anti-TNF Therapy in Crohn's Disease (PANTS) study followed about 1,600 patients with Crohn's disease, a form of IBD, for a year. It searched for genetic, clinical, serological, and microbiome markers associated with non-response to anti-TNF drugs. However, Barrett noted that a GWAS for primary non-response didn't find much of anything. He said he suspected that the phenotype of non-response might not be homogenous.
Because of that, they focused on a more specific phenotype of anti-drug antibodies. A GWAS looking at their development had a hit on chromosome six, at HLA-DQA1*05, he said.
When he and his colleagues examined the effect of this allele on the formation of antibodies, they found that 40 percent of patients without it form antibodies when on combination therapy, while those with one or more copies were even more likely to form antibodies, particularly if they were on a monotherapy.
This, Barrett said, demonstrates a clinically relevant correlation and that genetics can guide treatment approaches.
A 2017 study of 75,000 IBD patients linked about 250 loci to the condition. Barrett has since used statistical evidence of association between the SNPs identified and IBD to gauge how likely it is that that signal is the causal one. In that way, he said, the loci could be prioritized for follow up.
For these, he asked whether these genes could be causal, what the direction of their effect was, if there was therapeutic opportunity, what the mechanism of action might be, and whether there was an efficacy biomarker.
For instance, he found missense mutations in IL23R that appear causal. He also found that inhibiting IL23R appeared protective and appeared to decrease the pro-inflammatory signal. He also said that there is already a drug, Stelara (ustekinumab), that targets the ligand of IL23R.
"Genetics can clearly point to an opportunity for a drug," he said. However, he added, "the harder problem is to do it in a forward-looking way."
&
lt;br />
That, he said, is what he is now trying to do.
In particular, he has been investigating another locus with six SNPs that is annotated to ADAM15 or EFNA1. EFNA1, he noted, binds EphA2, and when he and his colleagues examined its binding affinity, they found that the variant turns binding off.
Meanwhile, they found that ADAM15 is associated with isoform usage and that the variant has an effect on splicing, and have uncovered two plausible functional variants.
Barrett is now drawing upon additional data, including from the UK Biobank, for fine mapping. Barrett said he suspects that there might be more than one causal variant that cannot be disentangled.
"The reductionist view of one variant and one gene likely [can led] us astray," he said.
Source: Wellcome Trusts Sanger Institute