German Study Finds That SARS-CoV-2 Mpro Reacts To Oxidation By Creating Disulfide And NOS/SONOS Bonds
Nikhil Prasad Fact checked by:Thailand Medical News Team May 09, 2024 11 months, 2 weeks, 2 days, 19 hours, 23 minutes ago
COVID-19 News: The COVID-19 pandemic brought into focus the importance of understanding the molecular mechanisms of SARS-CoV-2, particularly its main protease (Mpro, also known as nsp5 or 3CLpro). This enzyme plays a crucial role in viral replication by cleaving polyproteins into functional units necessary for the virus's survival and propagation. The significance of Mpro as a drug target led to extensive research efforts aimed at unraveling its structure and function.
 SARS-CoV-2 Mpro Reacts To Oxidation By Creating Disulfide And NOS/SONOS Bonds
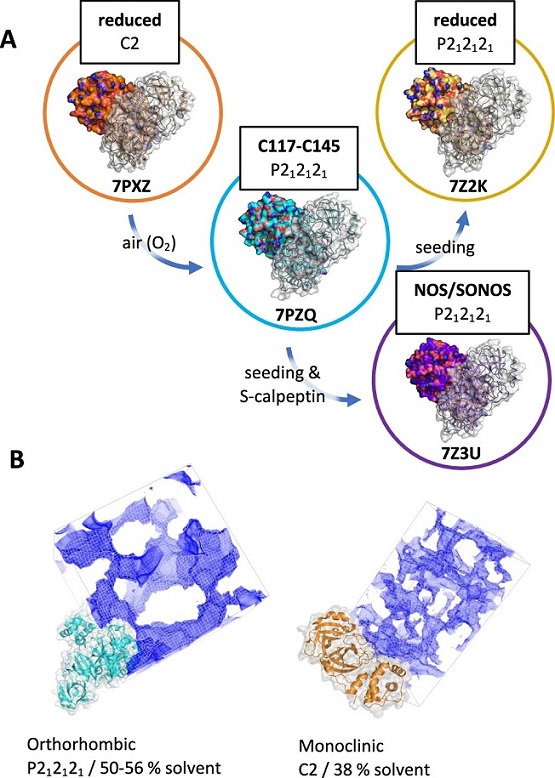
A Reduced protein under our crystallization conditions, containing TCEP, results in monoclinic (C2) protein crystals (PDB ID: 7PXZ). In a separate batch crystallization experiment, after oxidation by air and in the absence of reducing agent, the same protein spontaneously forms crystals with an orthorhombic (P212121) lattice and exhibits a disulfide link between C117 and C145 (PDB ID: 7PZQ). By producing seed crystals from this oxidized protein, however, we were able to obtain two further structures in the same orthorhombic lattice: first, using reduced protein, a structure with reduced C117/C145 (PDB ID: 7Z2K), and second, using the same reduced protein but with the addition of a sulfonated calpeptin ligand, a structure exhibiting NOS and SONOS crosslinks (PDB ID: 7Z3U). B Visualization of the orthorhombic and monoclinic lattices, with the solvent content highlighted by map-channels. The packing and crystal contact patterns are substantially altered, with the orthorhombic lattice exhibiting significantly larger solvent channels and an overall higher solvent content.
Mpro Regulation: Insights into Dimerization and Redox Sensitivity
SARS-CoV-2 Mpro Reacts To Oxidation By Creating Disulfide And NOS/SONOS Bonds
A Reduced protein under our crystallization conditions, containing TCEP, results in monoclinic (C2) protein crystals (PDB ID: 7PXZ). In a separate batch crystallization experiment, after oxidation by air and in the absence of reducing agent, the same protein spontaneously forms crystals with an orthorhombic (P212121) lattice and exhibits a disulfide link between C117 and C145 (PDB ID: 7PZQ). By producing seed crystals from this oxidized protein, however, we were able to obtain two further structures in the same orthorhombic lattice: first, using reduced protein, a structure with reduced C117/C145 (PDB ID: 7Z2K), and second, using the same reduced protein but with the addition of a sulfonated calpeptin ligand, a structure exhibiting NOS and SONOS crosslinks (PDB ID: 7Z3U). B Visualization of the orthorhombic and monoclinic lattices, with the solvent content highlighted by map-channels. The packing and crystal contact patterns are substantially altered, with the orthorhombic lattice exhibiting significantly larger solvent channels and an overall higher solvent content.
Mpro Regulation: Insights into Dimerization and Redox Sensitivity
Mpro's activity is intricately regulated through various mechanisms, including dimerization and sensitivity to the cellular redox environment. Dimerization, which occurs at higher enzyme concentrations, enhances Mpro's catalytic rate, highlighting a concentration-dependent regulatory mechanism. Structural studies have revealed unique features in Mpro's dimerization domain, emphasizing its role in enzyme regulation.
Furthermore, Mpro exhibits sensitivity to changes in the redox environment, with 12 cysteine residues in its sequence suggesting a potential regulatory role through oxidation-reduction reactions. Under reducing conditions, all cysteines are reduced, maximizing the enzyme's catalytic activity. Conversely, oxidative stress leads to diverse modifications, including disulfide bridges and other oxidative adducts.
The Impact of Oxidative Modifications on Mpro Function
A recent German study conducted by the Center for Free-Elec
tron Laser Science (CFEL), Deutsches Elektronen-Synchrotron (DESY), European XFEL GmbH, Universität Hamburg, and Max Planck Institute for the Structure and Dynamics of Matter that is covered in this
COVID-19 News report, sheds light on how oxidative modifications influence Mpro function. The study focused on understanding how oxidation affects Mpro's structure, activity, and potential implications for viral fitness.
The study's findings revealed a crucial disulfide bond between cysteines C117 and C145 in oxidized Mpro. This disulfide bridge was observed to protect the enzyme from irreversible oxidation, preserving its catalytic activity. Moreover, the study highlighted a correlation between oxidation and dimerization, with oxidized Mpro exhibiting weakened dimer stability compared to its reduced form.
Structural Insights: Disulfide Bond Formation and Dimer Stability
The study utilized advanced structural techniques, including crystallography, to investigate the effects of oxidation on Mpro's structure and function. By obtaining crystal structures of oxidized Mpro, the researchers were able to visualize the formation of a disulfide bond between C117 and C145. This bond was found to play a critical role in protecting the enzyme's active site from irreversible oxidative damage.
Furthermore, the study provided insights into how oxidation affects Mpro's dimerization. Mpro forms an obligate homodimer, and the presence of the C117-C145 disulfide bond disrupted interactions at the dimer interface, leading to decreased dimer stability. This observation suggests a direct link between oxidative modifications and changes in Mpro's quaternary structure, which in turn impact its catalytic activity.
Crystallographic Studies: Unraveling Redox-Dependent Crystallization Behavior
Crystallographic analyses provided additional insights into Mpro's redox-dependent behavior. The study observed distinct crystallization patterns between oxidized and reduced Mpro, indicating that the protein's redox state influences its crystalline structure. Specifically, oxidized Mpro exhibited a more loosely packed lattice compared to its reduced counterpart.
Seeding experiments with crystals from the oxidized state enabled the researchers to obtain structures with novel oxidative modifications. These modifications included cysteine-lysine-cysteine (SONOS) and lysine-cysteine (NOS) bridges, further highlighting the impact of oxidation on Mpro's structural diversity.
Implications for Drug Development and Viral Biology
The insights gained from this study have significant implications for drug development targeting SARS-CoV-2. Understanding how oxidative modifications influence Mpro's structure and activity is crucial for designing effective antiviral strategies. By targeting specific redox-sensitive sites on Mpro, researchers may develop therapeutics that can modulate viral replication and combat COVID-19.
Moreover, the study's findings shed light on the interplay between oxidative stress and viral fitness. Oxidative modifications of Mpro may play a role in viral adaptation to the host environment, influencing factors such as viral replication rates and immune evasion strategies. Further research in this area could uncover new avenues for disrupting viral biology and developing innovative antiviral interventions.
Conclusion
In conclusion, the German study provides valuable insights into the oxidative regulation of SARS-CoV-2 main protease (Mpro). By elucidating the structural and functional consequences of oxidation, the study enhances our understanding of viral biology and informs efforts to develop therapeutics targeting Mpro. Continued research in this area holds promise for advancing our knowledge of COVID-19 and paving the way for innovative antiviral interventions.
The study findings were published in the peer reviewed journal: Nature Communications.
https://www.nature.com/articles/s41467-024-48109-3
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/hyperactive-mutations-in-sars-cov-2-mpro-contribute-to-antiviral-drug-resistance
https://www.thailandmedical.news/news/covid-19-news-more-mpro-mutations-emerging-on-newer-sars-cov-2-variants-are-current-antivirals-still-effective
https://www.thailandmedical.news/news/breaking-news-study-finds-that-sars-cov-2-mpro-is-able-to-induce-lpcat3-cleavage,-providing-an-explanation-for-gastrointestinal-manifestations
https://www.thailandmedical.news/news/covid-19-new-american-researchers-uncover-the-importance-of-selenium-and-glutathione-supplementation-in-covid-19-as-sars-cov-2-mpro-targets-these
https://www.thailandmedical.news/news/study-discovers-that-sars-cov-2-mpro-protein-disarms-some-of-the-human-immune-responses-by-cleaving-nemo-an-immune-signaling-protein
https://www.thailandmedical.news/news/latest-danish-study-shows-that-mutations-e166v-and-l50f-e166v-on-sars-cov-2-variants-weakens-nirmatrelvir-a-component-of-paxlovid-mpro-binding
https://www.thailandmedical.news/news/breaking-german-scientists-discover-that-sars-cov-2-main-protease-mpro-causes-microvascular-brain-pathology
