Glaucoma News: American Study Uncovers The Role Of Mitophagy In Ocular Neurodegeneration
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 31, 2023 1 year, 5 months, 2 weeks, 6 days, 12 hours, 55 minutes ago
Glaucoma News: In the realm of medical research, the quest to unravel the mysteries of neurodegenerative diseases continues to yield fascinating insights. Among these conditions, glaucoma, age-related macular degeneration, and diabetic retinopathy are ocular neurodegenerative disorders that have been of particular interest. A recent study by researchers from the University of North Texas Health Science Center-USA has shone a spotlight on a cellular process called mitophagy and its potential role in these eye-related diseases. This
Glaucoma News report delves into the fascinating world of mitochondria, bioenergetics, reactive oxygen species, and the intricate dance of mitochondrial dynamics, fusion, and fission, all of which converge in the world of mitophagy. As we explore the intricate mechanisms, we'll uncover the potential implications for glaucoma and other ocular neurodegenerative diseases.
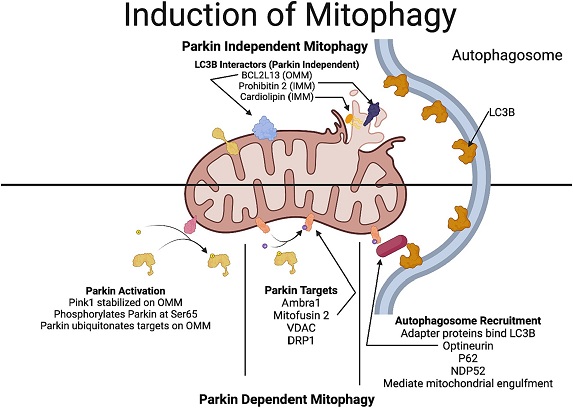 Mechanisms involved in Parkin-dependent and Parkin-independent mitophagy. In Parkin-dependent mitophagy, due to the decline in the mitochondrial potential, PINK1 gets stabilized in the outer mitochondrial membrane. PINK1 recruits Parkin (a ubiquitin ligase), which ubiquitinates several outer mitochondrial proteins including VDAC, mitofusin and DRP1. Optineurin and NDP52, which have a LC3-interacting region that act as preferential receptors for the ubiquitinated proteins to connect with the LC3 protein in the autophagosome membrane, thereby facilitating the engulfment of the mitochondria. In Parkin-independent pathway, proteins in the outer mitochondrial membrane, including, BNIP3, NIX/BNIP3L, FUNDC1, FKBP8, and Bcl2L13 which have a LC3-interacting region, directly interact with the autophagosome promoting the engulfment of the mitochondria by the autophagosome. Additionally, rupture of the outer mitochondrial membrane reveals more LC3-interactors such as prohibitin 2 and cardiolipin.
Introduction
Mechanisms involved in Parkin-dependent and Parkin-independent mitophagy. In Parkin-dependent mitophagy, due to the decline in the mitochondrial potential, PINK1 gets stabilized in the outer mitochondrial membrane. PINK1 recruits Parkin (a ubiquitin ligase), which ubiquitinates several outer mitochondrial proteins including VDAC, mitofusin and DRP1. Optineurin and NDP52, which have a LC3-interacting region that act as preferential receptors for the ubiquitinated proteins to connect with the LC3 protein in the autophagosome membrane, thereby facilitating the engulfment of the mitochondria. In Parkin-independent pathway, proteins in the outer mitochondrial membrane, including, BNIP3, NIX/BNIP3L, FUNDC1, FKBP8, and Bcl2L13 which have a LC3-interacting region, directly interact with the autophagosome promoting the engulfment of the mitochondria by the autophagosome. Additionally, rupture of the outer mitochondrial membrane reveals more LC3-interactors such as prohibitin 2 and cardiolipin.
Introduction
The complex world of ocular neurodegenerative diseases is one that scientists and researchers have been diligently exploring. Among these disorders, glaucoma takes center stage as a significant cause of blindness in the developed world, impacting millions of lives. The core of this condition's pathogenesis is the degeneration of the optic nerve and the loss of retinal ganglion cells (RGCs). In the search for answers, scientists have turned their attention to the mitochondria, the cellular powerhouses responsible for generating ATP, the primary energy source for cells.
Mitochondria and Bioenergetics
Mitochondria, often referred to as the power grid of eukaryotic cells, are responsible for producing ATP, the essential energy currency of cells. These organelles are equipped with an intricate inner membrane structure called cristae, which enhances their surface area, facilitating the electron transport chain. This chain is responsible for the oxidative metabolism of glucose and fatty acids, ultimately leading to ATP generation through oxidative phosphorylation.
However, this elegant process is not without its risks. As electrons traverse the electron transport chain, they can sometimes escape, leading to the production of reactive oxygen spe
cies (ROS). ROS, including superoxide radicals and hydrogen peroxide, can wreak havoc on the cell by damaging cellular components, particularly mitochondria.
While the body employs various antioxidants to keep ROS in check, an excess can lead to oxidative stress, contributing to various pathologies. Furthermore, certain tissues, such as the central nervous system (CNS), are more metabolically active and have a higher propensity for generating ROS. This includes the retina and optic nerve, which are among the most oxygenated and metabolically active tissues in the body.
The high metabolic demand of RGCs, which play a crucial role in vision, relies heavily on mitochondria to power essential cellular processes.
Excessive ROS production can lead to a decline in mitochondrial bioenergetics, a phenomenon linked to various neurodegenerative diseases, including Alzheimer's and Parkinson's disease. Mitochondrial mutations also play a role in ocular neurodegenerative disorders, such as Leber's Hereditary Optic Neuropathy (LHON). In these conditions, the retina and optic nerve heavily depend on oxidative phosphorylation for energy generation, which can result in optic nerve degeneration.
Mitochondrial Fusion and Fission
Mitochondria are dynamic organelles that respond to cellular stress and damage through a process called fusion. Fusion enables the mixing of mitochondrial components, allowing for genetic complementation to repair genetic defects caused by injury. Mitochondria also undergo fission, a process where they divide to meet increased energy demands or distribute to daughter cells during cell division. These dynamic processes create an interconnected mitochondrial network within cells.
Disruption in mitochondrial fusion and fission can lead to the loss of mitochondrial DNA, compromising bioenergetic functions. This disruption can be brought about by alterations in the expression of proteins involved in fusion (e.g., MFN1/2 and OPA1) and fission (e.g., Drp-1). Excessive fission has been associated with neurodegeneration, including glaucoma.
Mitophagy
Mitochondria can be damaged by various mechanisms, such as ROS overproduction, elevated Ca2+ levels, increased fission, and decreased fusion.
These insults can lead to the accumulation of damaged or dysfunctional mitochondria within cells, perpetuating damage through additional ROS production. To combat this, cells employ a quality control mechanism known as mitophagy. In mitophagy, damaged mitochondria are enveloped by a membrane, forming a double-membraned structure called a phagophore. This process involves ubiquitination reactions catalyzed by a group of proteins known as the ATG family.
In healthy mitochondria, PINK1 (PTEN-induced putative kinase 1) is imported into the inner mitochondrial membrane, where it is degraded. However, under conditions of oxidative stress and mitochondrial potential collapse, PINK1 stabilizes on the outer mitochondrial membrane, initiating a chain of events that recruit Parkin to ubiquitinate various mitochondrial proteins. These ubiquitinated proteins, in turn, recruit autophagy receptors like optineurin, which interact with the autophagosome membrane, facilitating the engulfment and subsequent degradation of damaged mitochondria within lysosomes.
Mitochondrial biogenesis, the process of generating new mitochondria, helps compensate for mitochondrial loss. This process is regulated by various transcription factors and coactivators like PGC-1α, NRF-1, NRF-2, and TFAM, which work together to regulate mitochondrial replication and repair. Maintaining a balance between mitophagy (to eliminate damaged mitochondria) and mitochondrial biogenesis (to generate new mitochondria) is crucial for a healthy mitochondrial network.
Mitophagy in Glaucoma
Glaucoma, a progressive neurodegenerative disease affecting the eyes, is a leading cause of blindness. The condition primarily damages the optic nerve and results in the loss of RGCs. Mitochondrial dysfunction has emerged as a significant player in the demise of RGCs in glaucoma. Studies have revealed that oxidative stress can lead to novel mutations in mitochondrial DNA, hinting at the role of mitochondrial damage in glaucoma. The aqueous humor and plasma of glaucoma patients have shown markers of oxidative stress, further linking mitochondrial dysfunction to the disease.
Animal models of glaucoma have consistently demonstrated that elevated intraocular pressure leads to increased mitochondrial fission, disrupted metabolism, and reduced respiratory capacity, all indicative of mitochondrial dysfunction. In a healthy scenario, mitophagy should clear damaged mitochondria, but the evidence suggests that mitophagy may be declining in glaucoma.
Some studies have shown an increase in mitophagy in animal models of glaucoma, indicating a potentially protective role. However, there is a discrepancy in findings that may be attributed to the specific animal model and the duration of the pathology. It's important to note that the process of mitophagy is dynamic, and more research is needed to understand the spatiotemporal changes in mitophagic flux in various glaucoma models.
Conclusion
The role of mitophagy in ocular neurodegeneration is a field that continues to evolve. While there is a wealth of data suggesting that a decline in mitophagy may contribute to neurodegenerative effects, there are also findings suggesting an increase in mitophagy in certain animal models of ocular neurodegeneration. The exact mechanisms leading to oxidative stress-mediated mitochondrial damage and the subsequent decline in mitophagy are still being unraveled.
Understanding these intricate pathways is crucial for gaining a deeper insight into mitophagic mechanisms and developing potential therapeutic targets for the prevention of neurodegeneration in various ocular neurodegenerative disorders. As research progresses, it is hoped that these discoveries will lead to new treatments that can safeguard vision and improve the lives of those affected by conditions like glaucoma, age-related macular degeneration, and diabetic retinopathy.
The study findings were published in the peer reviewed journal: Frontiers in Neuroscience.
https://www.frontiersin.org/articles/10.3389/fnins.2023.1299552/full
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
