Glaucoma News: Japanese Study Reveals Molecular Aspects Of Optic Nerve Autophagy In Glaucoma
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 17, 2023 1 year, 6 months, 3 days, 6 hours, 26 minutes ago
Glaucoma News: The optic nerve, a vital component of our visual system, is a complex structure composed of glial cells, blood vessels, and axons that include myelin and axoplasm. In glaucoma, a devastating eye condition, it's crucial to understand the molecular mechanisms underlying optic nerve degeneration, as axonal degeneration typically precedes the death of retinal ganglion cells. This
Glaucoma News report delves into a groundbreaking Japanese study conducted at St. Marianna University School of Medicine, Kanagawa, Japan, which explores the molecular aspects of optic nerve autophagy in glaucoma.
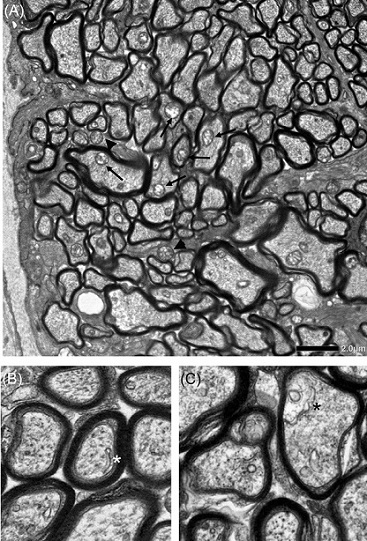 Electron microscopic findings 2 weeks after intravitreal injection of netarsudil +
Electron microscopic findings 2 weeks after intravitreal injection of netarsudil +
TNF in the optic nerve, when autophagy activation was detected. Autophagosomes
inside axons (black arrows) and in glia (black arrowheads) are shown (A). An isolated double
membrane (white asterisk) (B) and a phagophore (black asterisk) (C) inside axons were observed
approximately 1 mm behind the ocular globe. Scale bar = 2 μm (A) and 500 nm (B, C).
Glaucoma, often referred to as a neurodegenerative disease, is influenced by various factors, including elevated intraocular pressure (IOP), microvascular impairment, tumor necrosis factor (TNF) levels, oxidative stress, aging, genetic factors, axonal flow, collagen alterations, glial activation, and mitochondrial dysfunction. It is one of the leading causes of irreversible blindness worldwide, and its progression is closely linked to visual field defects, which correlate with optic nerve degeneration. While reducing IOP remains the standard treatment, a significant number of patients continue to experience disease progression despite achieving appropriate IOP levels. Consequently, there is a pressing need for IOP-independent therapies capable of halting the advancement of the disease.
The hallmark of glaucomatous histological changes is characterized by the loss of retinal ganglion cell (RGC) axons, ultimately leading to the death of these essential cells. Researchers have extensively explored the concept of neuroprotection in RGC death, and among various neuroprotective candidates, memantine, an N-methyl-D-aspartate (NMDA) receptor inhibitor, has been tested in large clinical trials, albeit with inconclusive results. It's crucial to understand that axonal degeneration takes place first in glaucoma, followed by retrograde RGC death. Therefore, protecting axons from degeneration is a viable approach to prevent further RGC loss.
Glaucoma and Autophagy
The relationship between autophagy and glaucoma has been a subject of controversy in the scientific community. Various studies employing pharmacological and genetic approaches have yielded inconsistent results regarding the role of autophagy in retinal ganglion cell (RGC) survival.
In pharmacological studies, both the autophagy inducer rapamycin and autophagy inhibitor 3-methyladenine (3-MA) have demonstrated protective effects on RGCs, adding to the uncertainty surrounding the role of autophagy. Similarly, genetic studies using Atg4B knockout mice, which exhibit reduced basal a
utophagy in the retinas, have shown susceptibility to RGC death following optic nerve injury, suggesting that autophagy is protective. However, other studies have reported that Atg4b deletion offers protection against optic nerve degeneration, indicating that autophagy deficiency can be protective.
These inconsistent findings reflect the complex interplay between autophagy and RGC survival, making it difficult to arrive at a conclusive understanding of the role of autophagy in RGC death. Nevertheless, most studies agree that there is an increase in autophagosomes in RGC bodies or their axons in various glaucoma models, although interpreting this increase is challenging because it doesn't always signify autophagy activation. Autophagosome accumulation can result from either increased autophagy or the impairment of autophagic flux, leading to differing interpretations.
Autophagy is a dynamic process, and understanding the autophagic flux in different disease models or clinical conditions is crucial. One key marker of autophagic flux is the protein LC3-II, which is reliably correlated with autophagosomes. However, interpreting LC3-II levels requires caution, as sensitivity to LC3-I and LC3-II may vary depending on the antibodies used. To gain a more comprehensive understanding of autophagic flux, LC3-II quantification should be complemented by other assays, such as the p62/SQSTM1 protein assay, as decreased p62 levels are associated with autophagy activation.
The connection between autophagy impairment and axonal degeneration is a common theme in various neurodegenerative diseases. Disrupted autophagy has been associated with dopaminergic axonal degeneration and pathological protein accumulation in Parkinson's disease. Conversely, enhancing autophagy has been shown to attenuate axonal degeneration, as seen in a Parkinson's disease model. Therefore, understanding the regulation of autophagy in axons is crucial for developing therapeutic strategies to protect and regenerate axons in neurodegenerative diseases, including glaucoma.
Regulation of Autophagy in Axons
Autophagy in axons is a dynamic and complex process, influenced by various molecular factors. Understanding the regulation of autophagy within axons is essential for developing strategies to protect and regenerate these crucial cellular structures.
1. Adenosine Monophosphate-Activated Protein Kinase (AMPK): AMPK is a critical kinase that acts as a sensor of energy, metabolism, and autophagy. It plays a role in regulating axonal autophagy. Activation of AMPK can both promote autophagy and influence axonal transport.
2. ULK1: ULK1 is a key regulator of autophagy and is directly phosphorylated by AMPK. Phosphorylation of ULK1 influences autophagosome biogenesis and maturation, making it a crucial player in the autophagy process within axons.
3. Nicotinamide Adenine Dinucleotide (NAD+): NAD+ synthesis pathways have been implicated in axonal protection. Reduced NAD levels have been observed in degenerating axons, and increasing NAD levels can promote axonal protection and autophagy activation.
4. Rho-Associated Protein Kinase (ROCK) Inhibition: Inhibition of ROCK has been linked to axonal regeneration and axonal protection. Recent studies suggest that ROCK inhibitors can promote autophagy, further contributing to axonal health.
5. Sirtuin 1 (SIRT1): SIRT1 is a histone deacetylase that regulates aging and plays a role in axonal protection and autophagy. Activation of SIRT1 has been shown to preserve axons and enhance autophagy in various neurodegenerative conditions.
6. p38 Inhibition: p38, a member of the mitogen-activated protein kinase (MAPK) family, is activated by environmental stress and inflammatory signals. Inhibition of p38 has been associated with axonal protection and autophagy enhancement, making it a potential therapeutic target for neurodegenerative diseases like glaucoma.
Conditions with Autophagy Enhancement in the Optic Nerve
Several conditions and interventions have been shown to enhance autophagy in the optic nerve, contributing to axonal protection and regeneration.
1. Hyperglycemia: Short-term hyperglycemia has been associated with enhanced autophagy, which can attenuate axonal degeneration and RGC death. However, long-term hyperglycemia can lead to autophagy dysregulation, contributing to RGC damage.
2. Calorie Restriction: Calorie restriction, known for its health-promoting effects, has been shown to activate autophagy in the optic nerve, promoting axonal health.
3. Exercise: Physical exercise can enhance autophagy in the optic nerve and contribute to axonal protection.
4. mTOR Inhibition: mTOR (mechanistic target of rapamycin) is a key regulator of autophagy. mTOR inhibition has been linked to axonal protection in various neurodegenerative conditions, including glaucoma.
5. Neurotrophic Factors: Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) have been shown to activate autophagy and promote axonal regeneration.
6. mGluR2 Activation: Activation of metabotropic glutamate receptor 2 (mGluR2) has been demonstrated to activate autophagy and protect axons in the optic nerve.
7. Antioxidants: Antioxidants can mitigate oxidative stress, promoting autophagy and axonal protection in glaucoma.
Concluding Remarks
Understanding the molecular aspects of optic nerve autophagy is essential for developing effective neuroprotective and regenerative strategies for glaucoma. Axonal degeneration is a crucial early event in glaucoma, and protecting axons from degeneration can prevent further retinal ganglion cell loss. Autophagy is a complex and dynamic process with varying implications for axonal health, and its precise role in glaucoma remains a subject of ongoing research.
While some interventions and conditions have been associated with autophagy enhancement in the optic nerve, it's crucial to tailor treatments based on individual patient needs and the specific stage of glaucoma. Future research efforts should focus on clarifying the intricacies of autophagy in glaucoma and developing targeted therapies to protect and regenerate axons, ultimately preserving vision for patients with this sight-threatening condition.
The study findings were published in the peer reviewed journal: Molecular Aspects of Medicine.
https://www.sciencedirect.com/science/article/pii/S0098299723000572
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
