Glaucoma News: Study Reveals The Critical Role Of Keratin 8 In Retinal Ganglion Cell Survival Under Acute Ocular Hypertension
Nikhil Prasad Fact checked by:Thailand Medical News Team Oct 14, 2023 2 years, 16 hours, 24 minutes ago
Glaucoma News: Glaucoma, a progressive optic neurodegenerative disease, remains a leading cause of irreversible blindness worldwide. Central to its pathogenesis is the loss of retinal ganglion cells (RGCs), primarily induced by pathologically high intraocular pressure (IOP). A recent study covered in this
Glaucoma News report, conducted at Zhejiang University in China has shed new light on the crucial role of Keratin 8 (KRT8) in preserving RGCs under acute ocular hypertension (AOH), providing invaluable insights into potential therapeutic strategies for glaucoma patients.
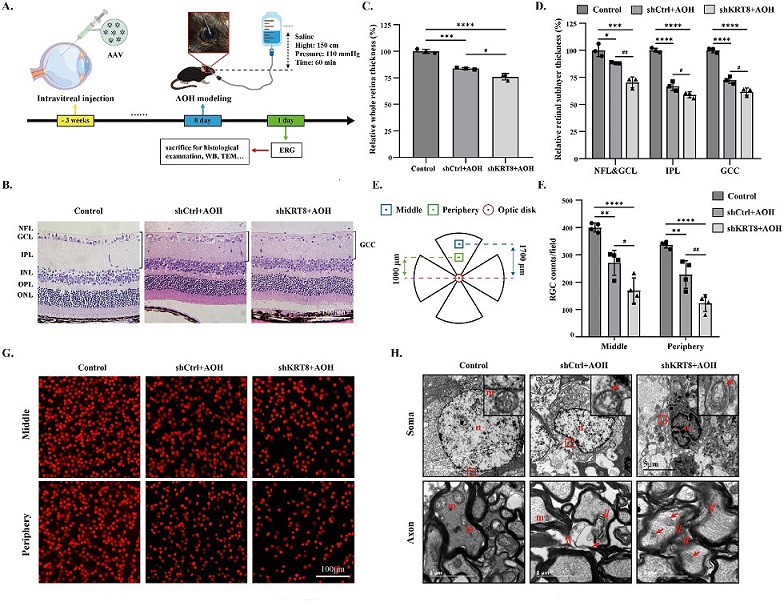 Keratin8 knockdown induced severer damage to RGCs in AOH retinas. (A) Schematic of the timeline and treatments of AAV intravitreal injection, AOH induction, and subsequent experiments. Note: Some used graphic materials are from the website of Figdraw. (B) Representative H&E staining images of retinas in different groups after AOH. Scale bar = 100 µm. (C, D) Quantitative analysis of the thickness of the whole retina and sublayers of the retinas after AAV injection through H&E staining images (n = 3). (E) Schematic of the middle and perpheiry areas of the retinal flat-mounts for the RGC counting. (F) Quantitative analysis of the Brn3a-positive RGC counts in the middle and peripheral areas of retinal flat-mounts (n = 4). (G) Representative immunofluorescence images of retinal flat-mounts in different groups after AOH, labeled with Brn3a (red). Scale bar = 100 µm. (H) Ultrastructural changes of RGC somas and axons in different groups after AOH. N = nuclei; m = mitochondria; red arrow = swollen or ruptured mitochondria; double red arrow = demyelination; red square = the area of each inset. Scale bar = 5 µm or 2 µm.
The Complexity of Glaucoma
Keratin8 knockdown induced severer damage to RGCs in AOH retinas. (A) Schematic of the timeline and treatments of AAV intravitreal injection, AOH induction, and subsequent experiments. Note: Some used graphic materials are from the website of Figdraw. (B) Representative H&E staining images of retinas in different groups after AOH. Scale bar = 100 µm. (C, D) Quantitative analysis of the thickness of the whole retina and sublayers of the retinas after AAV injection through H&E staining images (n = 3). (E) Schematic of the middle and perpheiry areas of the retinal flat-mounts for the RGC counting. (F) Quantitative analysis of the Brn3a-positive RGC counts in the middle and peripheral areas of retinal flat-mounts (n = 4). (G) Representative immunofluorescence images of retinal flat-mounts in different groups after AOH, labeled with Brn3a (red). Scale bar = 100 µm. (H) Ultrastructural changes of RGC somas and axons in different groups after AOH. N = nuclei; m = mitochondria; red arrow = swollen or ruptured mitochondria; double red arrow = demyelination; red square = the area of each inset. Scale bar = 5 µm or 2 µm.
The Complexity of Glaucoma
Glaucoma, characterized by the gradual loss of vision due to damage to the optic nerve, poses a significant challenge to both patients and healthcare providers. It is a multifactorial disease, with high IOP considered one of the primary risk factors.
However, the molecular mechanisms underlying RGC damage or survival in response to increased IOP are far from fully understood. AOH, akin to the ischemia-reperfusion model, simulates the pathogenesis of acute primary angle-closure glaucoma, where sudden, acute elevation of IOP leads to ischemic injury followed by RGC loss. It is under these conditions that researchers have started to explore the role of KRT8 in neuroprotection.
Keratin: More Than Just Structural Support
Keratins are key components of the intermediate filament family, forming the cytoskeletal structures vital for maintaining cell integrity and strength. These filaments require a pairing of type I and type II keratins to maintain stability. KRT8, also known as cytokeratin-8 (CK-8), plays a crucial role as a type II keratin, often paired with KRT18. In addition to providing mechanical support, previous research has unveiled nonmechanical functions of KRT8. These functions encompass the regulation of numerous physiological activities, including cell proliferation, differentiation, apoptosis, autophagy, endoplasmic reticulum (ER) stress, and sig
nal transduction. However, the study of KRT8 in neurocytes, particularly RGCs in the retina, remains relatively unexplored.
Discovering KRT8 in the Retina
The study identified the presence of KRT8/18 in retinas, with a significantly higher expression in RGCs compared to other retinal layers. In RGCs, KRT8/18 was localized in close proximity to Tuj-1, a marker of these cells. The expression of KRT8/18 was further found to increase following AOH induction in mouse retinas.
AAV-Mediated KRT8 Knockdown: A Key Experiment
To delve deeper into the role of KRT8 in RGCs, the researchers used an adeno-associated virus (AAV) system to selectively knock down KRT8 in these cells. This intervention led to a significant reduction in KRT8/18 expression, demonstrating the effectiveness of the technique. Importantly, this knockdown had no discernible effect on the retinal structure, thickness, RGC counts, or ultrastructural features under normal conditions. RGCs maintained their typical morphology, and no apoptotic cells were observed.
KRT8 Knockdown Exacerbates RGC Injuries
The true significance of KRT8 in RGCs became apparent when AOH was induced after KRT8 knockdown. In this scenario, AOH led to a more severe reduction in the thickness of the whole retina and inner retinal layers, including the nerve fiber layer and ganglion cell layer. The loss of RGCs was more pronounced, and ultrastructural changes, including nuclear and mitochondrial abnormalities, were exacerbated.
Visual function, as measured by the PhNR in electroretinography (ERG), was significantly impaired in the KRT8 knockdown group compared to the control group. This highlighted the critical role of KRT8 in preserving RGC function under high IOP conditions.
KRT8 Knockdown Promotes RGC Apoptosis and Glial Activation
RGC apoptosis was further confirmed through TUNEL assays, which revealed significantly more apoptotic cells in the KRT8 knockdown group after AOH induction. Western blot analysis showed a reduction in anti-apoptotic Bcl-2 and an increase in pro-apoptotic cleaved-Caspase 3 in AOH retinas with KRT8 knockdown.
The role of mitogen-activated protein kinase (MAPK) pathways was also investigated. The MAPK family comprises c-Jun N-terminal kinase (JNK), p38 MAPK, and extracellular signal-regulated protein kinase (ERK). In the shCtrl (control) group with AOH, an increase in phosphorylated JNK, p38, and c-Jun was observed, whereas phosphorylated ERK remained unaffected. KRT8 knockdown significantly intensified the phosphorylation of JNK, p38, and ERK, accompanied by elevated c-Jun expression.
Abnormal Activation of MAPK Pathways in RGCs
The aberrant activation of MAPK pathways was identified as a potential mechanism for the aggravated RGC damage observed in the KRT8 knockdown group. JNK and p38 activation is typically associated with apoptosis, while ERK activation tends to be neuroprotective. In the context of high IOP, AAV-mediated KRT8 knockdown intensified the phosphorylation levels of JNK and p38, as well as increased c-Jun expression, leading to RGC apoptosis.
However, the relationship between KRT8 and ERK was more complex, as KRT8 knockdown resulted in a decreased level of phosphorylated ERK. This could potentially be attributed to the pro-survival effect of ERK, which, when downregulated, might result in the activation of the NF-κB signaling pathway and increased gliosis. This, in turn, contributes to retinal neuroinflammation.
Conclusion- KRT8 as a Novel Neuroprotective Target
In conclusion, this groundbreaking study provides critical insights into the role of KRT8 in RGC neuroprotection under high IOP conditions, such as those found in glaucoma. The research demonstrated that KRT8 plays a pivotal role in preserving RGCs, and its deficiency leads to an abnormal activation of MAPK pathways, culminating in RGC apoptosis. These findings pave the way for further research into the underlying regulatory mechanisms and potential therapeutic approaches for glaucoma patients. The preservation of KRT8 in RGCs is a promising avenue for future investigations, offering hope for innovative treatments to safeguard RGCs and mitigate the devastating impact of glaucoma.
The study findings were published in the peer reviewed journal: Investigative Ophthalmology & Visual Science.
https://iovs.arvojournals.org/article.aspx?articleid=2792717&resultClick=1
For the latest
Glaucoma News, keep on logging to Thailand Medical News.
