GREAT NEWS! Study Shows That SARS-CoV-2 Protein Nsp13 Is Able To Cause DNA Damage And Dysregulate Tumor Suppressor Gene P53, Increasing Cancer Risk!
Source: SARS-CoV-2-DNA Damage Jun 14, 2022 3 years, 6 months, 1 week, 1 day, 4 hours, 19 minutes ago
SARS-CoV-2-DNA Damage: A new study by researchers from the Department of Genomic Medicine and Environmental Toxicology at the Universidad Nacional Autónoma de México has found that the SARS-CoV-2 protein Nsp13 is able to cause DNA damage and dysregulate tumor suppressor gene P53, increasing the risk of cancer to those who have been exposed to the protein! The study findings adds to the list of complications that can arise in Long COVID.
The COVID-19 caused by the SARS-CoV-2 coronavirus is challenging global health and economic systems. In some individuals, COVID-19 can cause a wide array of symptoms, affecting several organs, such as the lungs, heart, bowels, kidneys and brain, causing multiorgan failure, sepsis and death. These effects are related in part to direct viral infection of these organs, immunological deregulation, a hypercoagulatory state and the potential for development of cytokine storm syndrome.
Since the appearance of COVID-19 is recent, the long-term effects on the health of recovered patients remain unknown.
The
SARS-CoV-2-DNA Damage study team focused on current evidence of the mechanisms of DNA damage mediated by coronaviruses. Data supports that these viruses can induce DNA damage, genomic instability, and cell cycle deregulation during their replication in mammalian cells. Since the induction of DNA damage and aberrant DNA repair mechanisms are related to the development of chronic diseases such as cancer, diabetes, neurodegenerative disorders, and atherosclerosis, it will be important to address similar effects and outcomes in recovered COVID-19 patients.
The study findings were published in the peer reviewed journal: Mutation Research/ Reviews In Mutation Research by Science Direct (Elsevier).
https://www.sciencedirect.com/science/article/pii/S1383574222000011
The study team researchers described potential mechanisms of coronaviruses that could lead to deoxyribonucleic acid (DNA) damage and the potential link between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) activity and such mechanisms.
It is already known that COVID-19 patients may develop lymphopenia, elevated levels of alanine aminotransferase and C-reactive protein (CRP), and pulmonary alterations.
Cases of long COVID have increased, and COVID-19 symptoms have been reported to occur irrespective of COVID-19 severity. SARS-CoV-2 could become endemic to human beings, and therefore, understanding the long-term consequences of COVID-19 is extremely critical.
The study team explored the role of SARS-CoV-2 in the induction of DNA damage, which could contribute to the long-term consequences of COVID-19 in humans.
The novel SARS-CoV-2 coronavirus is highly homologous to the bat coronavirus RaTG13, and the receptor-binding motif (RBM) of the SARS-CoV-2 spike (S) protein, which recognizes human angiotensin-converting enzyme 2 (ACE2) receptor, shares high homology with pangolin coronaviruses. This indicates that SARS-CoV-2 probably originated from recombination between the pangolin and bat coronaviruses.
It is also known that the SARS-CoV-2 genome consists of open re
ading frames 1a and 1b (ORF1a and ORF1b), which translate into two polypeptide molecules, and their cleavage results in the formation of non-structural proteins (nsp).
Furthermore, the SARS-CoV-2 RNA serves as a template for viral replication mediated by RNA-dependent RNA polymerase (RdRp) nsp12.
The SARS-CoV-2 S1 RBM binds to ACE2 of the host, following which virions endocytosis and SARS-CoV-2 membrane fusion with the cell membrane takes place, releasing SARS-CoV-2 intracellularly. S protein cleavage by the transmembrane protease serine 2 (TMPRSS2) facilitates the endocytosis. Membrane fusion releases SARS-CoV-2 genomic RNA (gRNA) in the cytosol, facilitating SARS-CoV-2 genome replication.
Ways That Coronaviruses Directly Causes DNA Damage
It has been found that the Nsp13 protein from related coronaviruses such as SARS-CoV and infectious bronchitis virus (IBV) impair DNA polymerase δ activity, leading to DNA replication fork stress, DNA damage, histone, and H2AX (H2A histone family member X) phosphorylation, and arrest of the cell cycle.
As the nsp13 protein of SARS-CoV and SARS-CoV-2 are 99.8 % similar, this indicates that this mechanism could similarly be induced by the novel SARS-CoV-2.
It has also been found that SARS-CoV-2 infections induce overexpression of ATR (Ataxia telangiectasia and Rad3-related) and CHK1 (checkpoint kinase 1) and telomere shortening.
Just as alarming, the tumor suppressor gene p53 that primarily regulates the cell cycle, genomic stability, and the inhibition of replication of viruses is also affected by the SARS-CoV-2 Nsp13 protein which implications for risk of various cancers in those who had been infected with the SARS-CoV-2 virus!
It is well known that the Nsp3 protein encoded by SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and the human coronavirus (HCoV)-NL63 promotes p53 degradation and dysregulation.
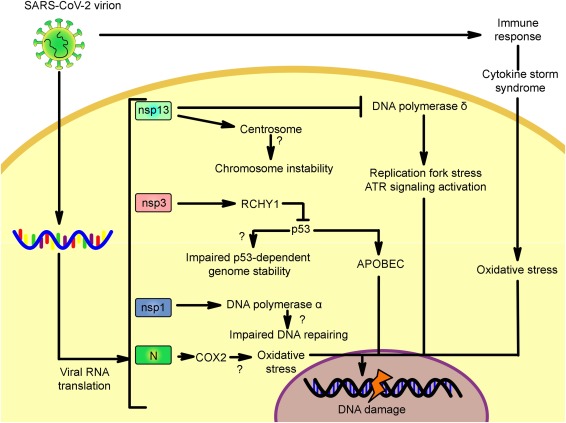 Potential mechanisms triggered directly by SARS-CoV-2 proteins and indirectly through the immune system. The mechanisms are based on the knowledge of proteins encoded by the SARS-CoV-2, SARS-CoV. Infectious bronchitis virus and the MERS-CoV. Since the orthologous proteins encoded in the SARS-CoV-2 genome share a high percentage of identity/similarity, it is possible that these mechanisms are induced by this newly described virus. Nsp13: non-structural protein 13, nsp3: non-structural protein 3, nsp1: non-structural protein 1, N: nucleocapsid, RCHY1: ring-finger and CHY zinc-finger domain-containing 1 ubiquitin ligase, Cox2: cyclooxygenase 2, APOBEC: apolipoprotein B mRNA editing enzyme catalytic polypeptide-like family of cytidine deaminases.
Potential mechanisms triggered directly by SARS-CoV-2 proteins and indirectly through the immune system. The mechanisms are based on the knowledge of proteins encoded by the SARS-CoV-2, SARS-CoV. Infectious bronchitis virus and the MERS-CoV. Since the orthologous proteins encoded in the SARS-CoV-2 genome share a high percentage of identity/similarity, it is possible that these mechanisms are induced by this newly described virus. Nsp13: non-structural protein 13, nsp3: non-structural protein 3, nsp1: non-structural protein 1, N: nucleocapsid, RCHY1: ring-finger and CHY zinc-finger domain-containing 1 ubiquitin ligase, Cox2: cyclooxygenase 2, APOBEC: apolipoprotein B mRNA editing enzyme catalytic polypeptide-like family of cytidine deaminases.
Since the Nsp3 protein of SARS-CoV and SARS-CoV-2 is similar, the degree of p53 dysregulation could also be similar between the viruses. The degradation of p53 could deregulate the APOBEC3B (apolipoprotein B mRNA editing enzyme catalytic subunit 3b) gene and cytidine deaminase enzymes, resulting in genomic instability.
The SARS-CoV nucleoprotein (N) induces the overexpression of the cyclooxygenase-2 (COX2) in pulmonary cells and contributes to the transcription and binding of NF-κB (nuclear factor kappa B) and C/EBP (CCAAT-enhancer-binding proteins) binding regions of the COX2 promoter. COX2 enhances genetic instability and DNA injury by the induction of DNA adducts and by influencing the cellular expression of glutathione.
In addition, COX2 leads to prostaglandin E2 production, which enhances the pro-inflammatory state and can lead to exacerbations of pro-oxidant conditions and induce DNA damage.
Interestingly, the Nsp1 protein can also interact with DNA polymerase α enzyme subunits, thus altering DNA synthesis and regulation of cell cycle, and can impair DNA repair processes.
Also, Nsp13 of SARS-CoV-2 has been found to interact with multiple centrosome proteins and disrupt centrosome duplication and/or structure can give rise to genomic instability by facilitating aneugenic pathways, which result in alterations in chromosome structure and number.
Other Indirect Ways That SARS-Cov-2 Can Cause DNA Damage
It has also been found that COVID-19 severity is characterized by overexpression of interleukin-6 (IL-6) and CRP levels. High CRP levels have been associated with increased oxidative damage in the DNA of individuals with obesity, psoriasis, obesity, cardiovascular disorders, and pancreatic cancer. Chronic and aberrant inflammation also leads to reactive oxygen species (ROS) overexpression. Individuals with severe SARS-CoV-2 infections have a pro-inflammatory and pro-oxidative state.
It has also been discovered in SARS-CoV-2-positive individuals that serum glutathione levels and total thiol levels are reduced. In contrast, catalase superoxide dismutase (SOD) and malondialdehyde (associated with lipid peroxidation and oxidative stress) levels and the total oxidant levels are increased with a substantial upregulation or increase in pro-oxidant gene expression, primarily the calprotectin myeloperoxidase (MPO) and genes.
It is well known that increased levels of oxidative stress can induce various types of DNA injury, such as breaks in single- and double strands of DNA, cross-linkage of DNA and proteins, and the formation of several products of oxidation of nucleic acid constituent bases and sugars, for example, the generation of guanine oxidized species (GOS).
The study findings highlight the possibilities for SARS-CoV-2 to impair DNA repair mechanisms, induce DNA damage, and induce oxidative stress in human cells and show that COVID-19 can be a multisystemic disease that could have long-lasting effects on the affected organs.
More importantly, oxidative DNA damage has long been associated with increased risks of developing neurodegenerative diseases and several types of cancers.
This raises the possibility that severe COVID-19 patients, who have a pro-inflammatory and pro-oxidative states, could also have higher risks of developing other chronic diseases in the long term, and that perhaps in cases of patients with comorbidities, these comorbid diseases could be worsened by SARS-CoV-2 viral infection.
Urgent detailed studies are needed to address the risks of developing such pathologies in both ailing and recovered COVID-19 patients.
For more on
SARS-CoV-2 And DNA Damage, keep on logging to Thailand
Medical News.

