Herbs And Phytochemicals: Polysaccharide Extracts Of Prunella Vulgaris Inhibits Herpes Simplex Virus Infection By Blocking TLR-Mediated NF-kB Activation
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 12, 2024 1 year, 3 months, 5 days, 22 hours, 43 minutes ago
Herbs And Phytochemicals: In recent years, traditional Chinese medicine has been at the forefront of research due to its holistic approach to treating various ailments. Among the myriad of medicinal herbs, Prunella vulgaris, an herbaceous plant from the Labiatae family, has emerged as a subject of interest. The Shanghai University of Traditional Chinese Medicine conducted an extensive study, revealing the potent antiviral effects of Prunella vulgaris polysaccharide extracted through hot water and 30% ethanol precipitation (PVE30) against herpes simplex virus (HSV) infections. This
Herbs And Phytochemicals news report delves into the comprehensive findings of this study, shedding light on the multifaceted mechanisms that make PVE30 a promising candidate for antiviral therapies.
 Prunella Vulgaris Inhibits Herpes Simplex Virus
Background - The Rich Pharmacological Portfolio of Prunella Vulgaris
Prunella Vulgaris Inhibits Herpes Simplex Virus
Background - The Rich Pharmacological Portfolio of Prunella Vulgaris
Prunella vulgaris has been a staple in traditional Chinese medicine, known for its diverse pharmacological properties. Extracts from P. vulgaris, particularly PVE30, have demonstrated potential in various therapeutic areas, including antitumor, anti-inflammatory, antioxidative, and antiviral effects. While previous research hinted at the antiviral properties, this study aims to provide a more nuanced understanding by unraveling the mechanisms behind PVE30's efficacy against HSV infections.
Understanding Herpes Simplex Virus
Herpes simplex viruses, HSV-1 and HSV-2, are ubiquitous human pathogens responsible for oropharyngeal and genital infections. The initial steps of HSV entry into cells involve the binding of viral envelope glycoproteins to heparan sulfate (HS) receptors on the cell surface. Targeting this crucial interaction holds immense potential for developing antiviral drugs.
Mechanisms of Action Explored
-Inhibiting Viral Attachment and Penetration
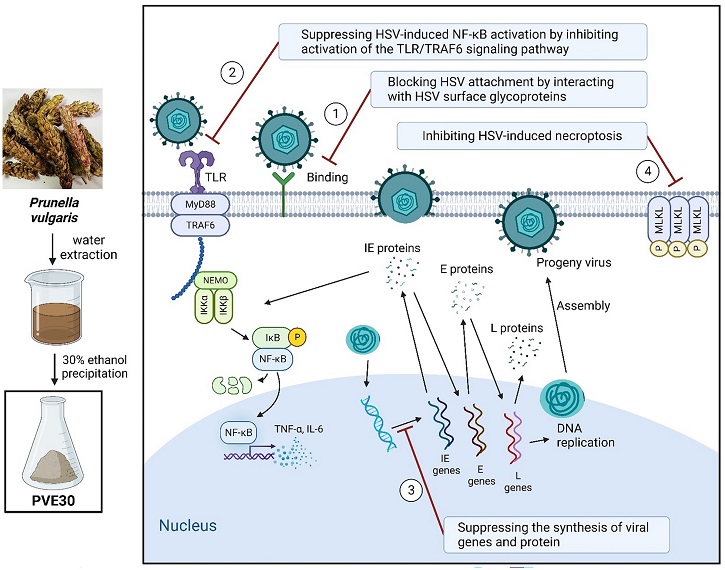
PVE30 demonstrated a direct impact on virions by interacting with HSV surface glycoproteins B and gC. These interactions hindered the virus's ability to attach to host cells, providing a robust defense against viral entry. By competing with HS, a cellular glycosaminoglycan, PVE30 significantly reduced the efficiency of virus attachment, offering a dual-pronged approach to inhibit viral penetration.
-Restricting Viral Replication
Beyond hindering viral entry, PVE30 exhibited a profound impact on viral replication. The study identified a downregulation in the expression of immediate early (IE) genes, particularly UL54, leading to decreased levels of the IE gene product ICP27. This downregulation impeded the precursor mRNA splicing and nuclear export of viral transcripts, effectively limiting the expression of early (E) and late (L) viral gene products and reducing the yield of progeny virus.
-Suppressing TLR-Mediated NF-κB Activation
Toll-like receptors (TLRs) play a pivotal
role in recognizing viral proteins and nucleic acids, initiating host defense mechanisms. PVE30 demonstrated the ability to inhibit TLR2 and TLR3 signaling, effectively suppressing the activation of nuclear factor-kappa B (NF-κB). NF-κB, a transcriptional regulator, plays a key role in regulating host gene expression, influencing cell survival, differentiation, inflammation, and antiviral responses. By curbing NF-κB activation, PVE30 hindered HSV replication at a fundamental level.
-Blocking HSV-1-Induced Necroptosis
PVE30 showcased its versatility by preventing HSV-1-induced necroptosis, a form of programmed cell death triggered by viral dsRNA intermediates. By reducing the phosphorylation of mixed lineage kinase domain-like (MLKL), a key player in necroptosis, PVE30 maintained a delicate balance between cell death, proliferation, and differentiation during HSV-1 replication.
The Complex Interplay - Exploring the Interactions
To further understand PVE30's intricate mechanisms, the study investigated the direct interactions between PVE30 and HSV glycoproteins. Through heparin bead pull-down assays, it was confirmed that PVE30 competes with HS for interaction with viral glycoproteins B and gC. This competition resulted in a strong inhibition of HSV attachment to cells, reinforcing the critical role of PVE30 in disrupting the initial stages of viral infection.
 Graphical Abstract
Elucidating Viral Gene Expression - Unraveling the Molecular Cascade
Graphical Abstract
Elucidating Viral Gene Expression - Unraveling the Molecular Cascade
The study employed real-time PCR to delve into the molecular cascade triggered by PVE30. It was found that PVE30 not only downregulated the expression of IE genes but also exerted downstream effects on the expression of E and L viral gene products. This comprehensive downregulation effectively restricted the yield of progeny virus, highlighting the broader impact of PVE30 on the entire virus replication cycle.
NF-κB Pathway Inhibition - A Crucial Defense Mechanism
The persistent activation of NF-κB, induced by HSV infection, enhances virus replication. PVE30's ability to alleviate HSV-1-triggered NF-κB activation was investigated in detail. The study revealed that PVE30 achieved this by inhibiting IKKβ phosphorylation, upregulating IκBα expression, and downregulating p65 expression and nuclear translocation. Additionally, PVE30 reduced the secretion of downstream inflammatory cytokines IL-6 and TNF-α, further emphasizing its role in modulating the host response to HSV infection.
Navigating the Terrain of Necroptosis - MyD88-Independent TLR Signaling
PVE30's impact on HSV-induced necroptosis was explored, shedding light on its ability to decrease the percentage of necroptotic cells and reduce p-MLKL expression. Interestingly, the study suggested that PVE30 may inhibit HSV-induced necroptosis through the TLR3 signaling pathway, recognized as a MyD88-independent TLR signaling pathway. This finding adds another layer to the multifaceted nature of PVE30's antiviral mechanisms.
Conclusion - PVE30 as a Multifaceted Antiviral Agent
In conclusion, the study conducted at the Shanghai University of Traditional Chinese Medicine provides a comprehensive understanding of Prunella vulgaris polysaccharide extract PVE30 as a potent antiviral agent against HSV infections. Its multifaceted mechanisms, including direct inactivation of virions, inhibition of viral replication, suppression of TLR-mediated NF-κB activation, and prevention of HSV-1-induced necroptosis, position PVE30 as a promising candidate for the development of antiviral therapies. This research not only validates the traditional uses of Prunella vulgaris but also opens new avenues for further exploration and pharmaceutical development in the realm of antiviral treatments.
The study findings were published in the peer reviewed journal: Chinese Medicine.
https://cmjournal.biomedcentral.com/articles/10.1186/s13020-023-00865-y
For the latest on
Herbs And Phytochemicals, keep to Thailand Medical News.

