HIV News: New Study Unravels the Intricate Pathway Of HIV Invasion Into The Cell Nucleus - A Glimpse Into New Avenues For AIDS Treatment
Thailand Medical News Team Aug 10, 2023 2 years, 4 months, 2 weeks, 4 days, 3 hours, 2 minutes ago
HIV News: In a groundbreaking study published on August 10, 2023, in the prestigious journal Nature Communications, scientists have unveiled a novel and previously undiscovered route through which the human immunodeficiency virus (HIV) infiltrates the nucleus of healthy cells, thereby initiating a cascade of events that can lead to the replication of the virus and subsequent infection of other cells. This new research not only sheds light on the intricate mechanisms of HIV invasion but also introduces promising avenues for potential drug development that could revolutionize the treatment of acquired immune deficiency syndrome (AIDS).
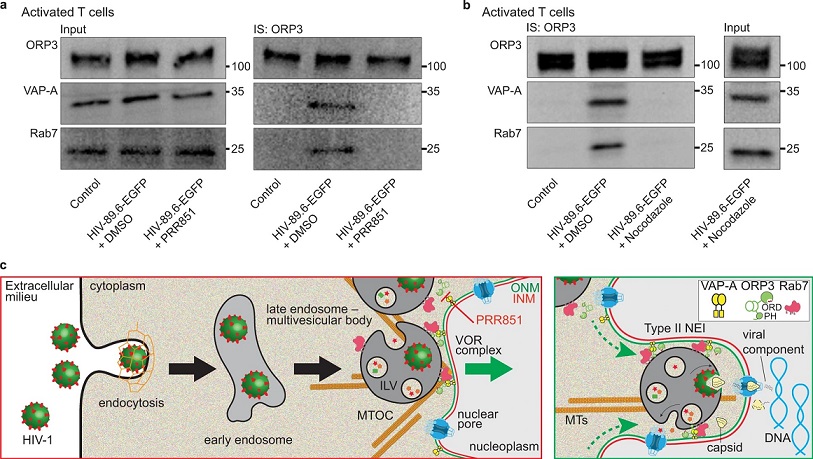 HIV-1 infection promotes the VOR complex formation in a microtubule-dependent manner.
HIV-1 infection promotes the VOR complex formation in a microtubule-dependent manner.
a PHA/IL-2-activated CD4+ T cells were noninfected (control) or 6-h HIV-89.6-EGFP infected after 30-min pretreatment with DMSO or 10 µM PRR851. The drug was present during infection. Cells were then solubilized and subjected to ORP3 IS. The bound fractions and the input (1/50) were probed for ORP3, VAP-A, and Rab7 by IB. b Cells were 5-min pretreated with DMSO or nocodazole (1 µM) and then noninfected (control) or 1-h HIV-89.6-EGFP infected, solubilized and processed for ORP3 IS and ORP3, VAP-A and Rab7 IB. All samples came from the same experiment and were run in parallel (a, b). Molecular mass markers (kDa) are indicated. Representative experiments are shown. We used a MOI of 2. Note that VOR complex formation only occurs after HIV-1 infection and is hindered by PRR851 and nocodazole. c Representation of the induction of type II NEIs by virus-laden late endosomes, a process mediated by the interaction of VOR complex proteins, namely ONM-associated VAP-A, cytoplasmic ORP3, and late endosome-associated Rab7 (left panel). Release of viral components from late endosomes into the cytoplasmic core of induced NEIs at the vicinity of the nuclear pore would facilitate their transfer to the nucleoplasm (right panel). PRR851 inhibits the interaction of the VOR complex proteins, and hence the NEI formation. ILV, intralumenal vesicles associated with late endosome/multivesicular body; INM/ONM, inner/outer nuclear membrane; MTOC, microtubule-organizing center; MTs, microtubules.
HIV infection, a global health concern that has defied a complete cure, hinges on the virus's ability to infiltrate and commandeer the nucleus of host cells for its replication. Despite significant progress in understanding various stages of the virus's life cycle, the precise mechanisms by which it breaches the protective fortress of the nucleus have remained enigmatic, fueling ongoing debates and posing challenges for therapeutic intervention as covered in various past studies and HIV News reports.
The recent study, led by senior author Dr Aurelio Lorico, MD, Ph.D., a distinguished Professor of Pathology and interim Chief Research Officer at Touro University Nevada College of Osteopathic Medicine, has brought to light a groundbreaking pathway through which HIV gains access to the cell nucleus. This newfound pathway centers around the utilization of endosomes, membrane-bound packages that transport substances within cells. The virus enters the host cell encased in an endosome, which then induces an astonishing alteration in the nuclear membrane - a phenomenon known as "nuclear invagination." This inward folding of the protective nuclear envelope creates a conducive environment for
the virus to traverse into the nucleus.
The study's significance lies not only in uncovering this novel pathway but also in identifying three crucial proteins that orchestrate the viral invasion. The first protein, Rab7, resides on the endosome's membrane, facilitating its interaction with the second protein, VAP-A, positioned on the nuclear membrane at the site of invagination. The third protein, ORP3, acts as a bridge connecting Rab7 and VAP-A, thus orchestrating a synchronized molecular dance that culminates in successful viral entry into the nucleus.
Crucially, the researchers have capitalized on this newfound knowledge to synthesize molecules that target the interactions among these pivotal proteins. Their experiments demonstrated that in the presence of these synthesized molecules, HIV replication is stifled, potentially paving the way for innovative therapeutic strategies to combat AIDS.
Dr Lorico told
HIV News reporters at Thailand Medical News, "This is an entirely new pathway, and we have developed molecules (drugs) that block it."
While acknowledging that the research is at a pre-clinical stage, he highlights the potential application of these novel drugs not only in AIDS treatment but also in addressing other viral diseases. The implications extend further, encompassing diseases like metastatic cancer, where nuclear transport plays a crucial role. The research team is actively investigating the involvement of this pathway in Alzheimer's disease and the metastasis of various cancer types, unraveling new avenues for targeted therapies.
Dr Denis Corbeil, a co-leading author of the study and research group leader at the Biotechnology Center (BIOTEC) of TUD Dresden University of Technology in Germany, underscores the broad impact of this discovery, emphasizing its applicability to a myriad of diseases. "Because the pathway we found may apply to many types of disease, there is a tremendous amount of work that needs to be done to understand the full benefits of this research," he asserts.
The implications of this research extend beyond the laboratory, resonating with Touro University's mission to serve humanity. Dr Alan Kadish, President of Touro University, lauds the research's potential therapeutic applications and its alignment with the institution's commitment to advancing patient care. He reflects on the profound impact of this discovery in navigating future pandemics and elevating patient well-being.
The study, titled "HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells," stems from a collaborative effort involving researchers from diverse institutions, including Touro University Nevada College of Osteopathic Medicine, Touro College of Osteopathic Medicine in New York, the Biotechnology Center (BIOTEC) of TU Dresden University of Technology in Germany, and researchers from Italy.
https://www.nature.com/articles/s41467-023-40227-8
The significance of this research becomes even more apparent when delving into the intricate molecular underpinnings of the newly discovered pathway. The study reveals that the process of HIV-1 nuclear entry is far from linear; rather, it involves a series of complex interactions and coordinated steps. This understanding challenges previous assumptions about viral entry and could reshape our strategies for combating HIV.
Historically, the cellular entry of HIV-1 has been a subject of intense investigation. While some studies suggested direct fusion with the plasma membrane, accumulating evidence pointed towards a different route – fusion with endosomal membranes upon cellular uptake. The present study builds upon this notion, demonstrating that HIV-1, pseudotyped with different envelope proteins, exploits the endocytic pathway for cellular entry. This pivotal discovery has far-reaching implications for our understanding of retroviral entry mechanisms.
The intricate web of interactions involves a trio of proteins - Rab7, VAP-A, and ORP3-each playing a distinctive role in orchestrating the invasion. The first, Rab7, is situated on the endosome's membrane, while VAP-A resides on the nuclear membrane. ORP3, the third protein, bridges the gap between Rab7 and VAP-A, creating a complex that propels the endosome towards the nuclear membrane, ultimately resulting in nuclear invagination. Disrupting any component of this protein trio can halt the invasion process, presenting an array of potential targets for therapeutic intervention.
The researchers' approach to drug development capitalizes on this newfound knowledge, as they synthesized molecules that impede the interactions among these proteins. In a compelling demonstration of the potential of these molecules, HIV replication was successfully inhibited in their presence. This breakthrough paves the way for novel antiretroviral treatments that target this intricate invasion pathway.
The study's impact reverberates far beyond the realm of HIV. Drawing parallels between the newly uncovered pathway and their previous research on cancer metastasis, the scientists suggest that similar mechanisms could be at play in various diseases. This revelation opens up exciting prospects for addressing a spectrum of diseases that hinge on nuclear transport, ranging from viral infections to metastatic cancer.
As the scientific community delves deeper into the implications of this research, the promise of innovative therapies comes into sharper focus. While these findings mark a significant step forward, the road ahead is rich with possibilities. Continued research into the intricate interactions among these proteins and their implications for various diseases holds the potential to reshape medical interventions and revolutionize patient care.
In conclusion, the study's findings mark a pivotal moment in our understanding of HIV invasion and nuclear entry. By unraveling the complex dance of proteins that underlie this process, researchers have not only shed light on a previously enigmatic mechanism but also paved the way for groundbreaking therapeutic strategies. The intersection of virology, cellular biology, and drug development holds tremendous potential for the future of AIDS treatment and beyond, offering a glimpse into a new era of medical innovation. As the scientific community continues to probe the depths of this newfound knowledge, the prospect of more effective and targeted treatments for a range of diseases is becoming an ever more tangible reality.
For the latest
HIV News, keep on logging to Thailand Medical News.
